Figure 1.
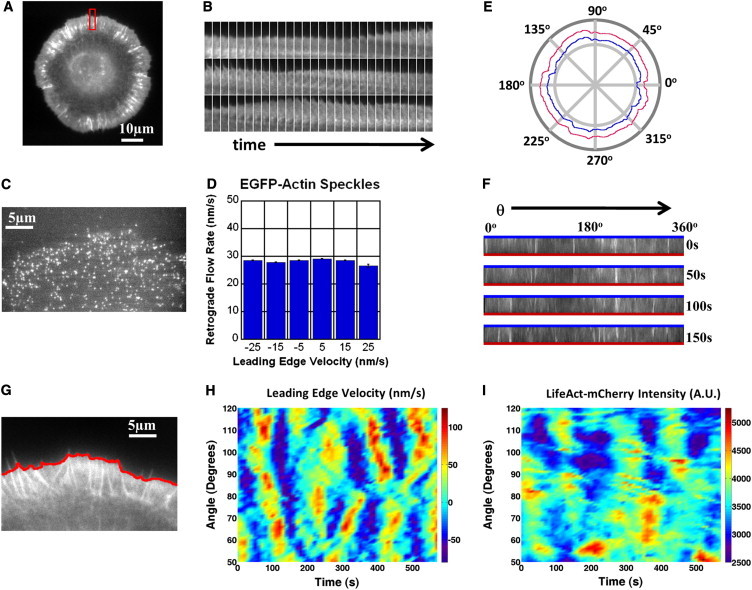
Leading-edge velocities and LifeAct intensity measured by fitting active contours to the leading edge. (A) XTC cell expressing LifeAct-mCherry. (B) Kymograph of leading edge (10 s intervals) for indicated section in panel A. (C) XTC cell expressing EGFP-actin in low concentration. (D) Actin speckles within 5 μm of the leading edge of the cell in panel C exhibit small variations in retrograde flow rate compared to variations in leading-edge speed. Bars indicate mean ± SE. (E) Polar coordinate system indicating the leading edge and the inner boundary of a 4-μm-wide band of lamellipodium. (F) 4-μm-wide bands of lamellipodium, mapped from a two-dimensional ribbon of lamellipodium. The lines indicate the inside and outside of the cell as in panel E. (G) Section of XTC cell expressing LifeAct-mCherry with superimposed active contour. Cell has been on the substrate for 40 min. (H) Normal leading-edge velocity (with respect to fixed substrate) versus angle and time, for cell in panel G. Positive (negative) velocities indicate protrusion (retraction). The retrograde flow speed for this cell (74 ± 3 nm/s, n = 15 measurements of bright features in kymographs) did not vary noticeably during observation. (I) Total LifeAct-mCherry intensity versus angle and time. LifeAct-mCherry intensity was summed over a 5-μm ribbon of lamellipodium of cell in panel G for each time point.
