Abstract
An osteosarcoma developed in the tarsal joint region involving the distal tibia of a domestic rabbit (Oryctolagus cuniculus). Micrometastases were present in the lungs. Histologically the tumor was composed of ovoid to short-spindle cells with abundant giant cells, producing irregular islands of osteoids. The tumor cells were immunopositive with antiosteocalcin monoclonal antibody, consistent with their derivation from osteoblasts. According to review of 10 published cases, productive osteoblasic osteosarcoma is the most common bone tumor in rabbits, with half of all cases developing in the skull or facial bones.
Osteosarcomas are malignant neoplasms that are known to occur in most species; in humans, these lesions are the most common primary bone tumor (excluding hematopoietic intraosseous tumors) in adolescents and young adults.7 Osteosarcoma accounts for more than 70% of malignant bone tumors in cats and 80% of those in dogs.2,16 Osteosarcoma rarely arises spontaneously in either domestic or experimental rabbits.1,5,10,13,14 We recently reported a rabbit that had an osteosarcoma of the right glenohumeral joint.9 Several cases of rabbit osteosarcoma have been published, but a comprehensive study has not been reported. Here we describe an additional case of osteosarcoma in a rabbit and review the sites, biologic behavior, and histopathologic types of osteosarcoma in rabbits.
Case Report
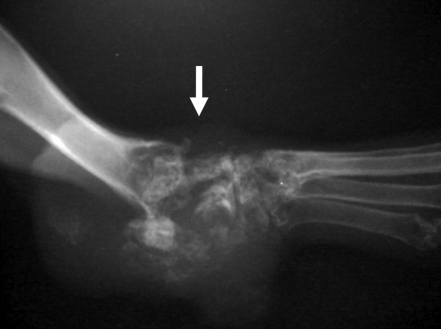
A 7-y-old, female, crossbred pet rabbit weighing 2.6 kg was referred by a local veterinarian with the complaint of swelling and bleeding in the tarsal region of the right hindlimb. A mass about 1 cm in diameter was present in the tarsal joint. Radiographs revealed bone lysis and hyperplasia extending to the distal tibia and calcaneal tuberosity (Figure 1), subluxation of digital joints of the forelimb, swelling of mediastinal lymph nodes, and liver enlargement. Lung metastasis was not detected on the radiograph. The serum ALP level was 147 U/L. The mass was excised by CO2 laser under inhalation anesthesia, but 3 d later the rabbit developed respiratory failure and died. Necropsy was performed at the local hospital, and tissue samples including the tumor mass, lungs, and liver were submitted to Nihon University for histopathologic examination. The rabbit had a past history of uterine adenocarcinoma and leiomyoma.
Figure 1.
A mass developed in the tarsal joint region (arrow) of the right hindlimb. Bone lysis and hyperplasia were present in the mass.
The removed tissues were fixed in 10% neutral buffered formalin, and paraffin-embedded samples were sectioned at 5 μm. The thin sections were stained with hematoxylin and eosin and Masson trichrome for light microscopic examination. Immunohistochemistry was performed by using the streptavidin–biotin–peroxidase method with mouse antihuman cytokeratin monoclonal antibody, mouse antihuman vimentin monoclonal antibody (Dako Japan, Tokyo, Japan), and mouse antibovine osteocalcin monoclonal antibody (Cosmo Bio LSL, Tokyo, Japan).
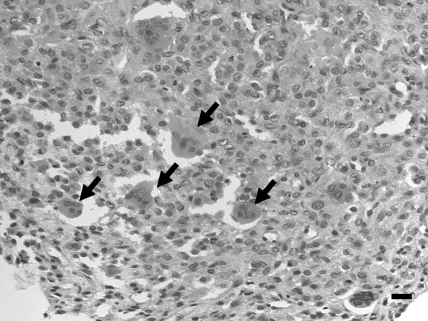
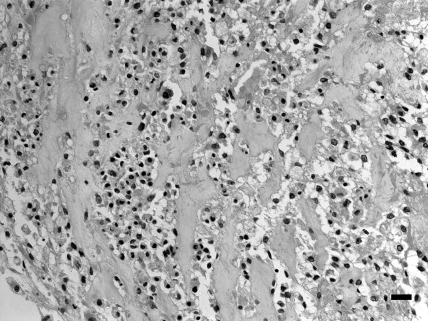
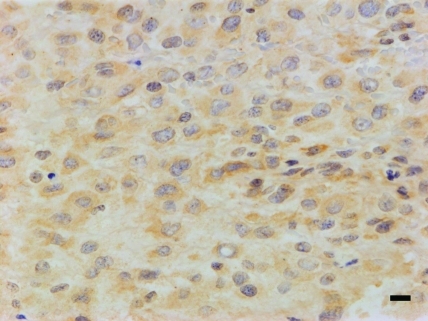
Grossly, serous fluid had accumulated in the pleural cavity and bloody fluid in the abdominal cavity. The lungs contained scattered gray spots measuring 0.5 to 1 mm in diameter. The liver appeared yellowish in color. Histologically, the mass of the right tarsal joint was composed of ovoid to short-spindle cells with atypical nuclei containing a moderate amount of chromatin and eosinophilic cytoplasm. Numerous mitotic figures and multinucleated giant cells were present (Figure 2). Neoplastic cells were located around eosinophilic and amorphorus osteoid in the intercellular matrix (Figure 3), which stained positively with Masson trichrome. Fibrovascular proliferation was noted in these areas. Small foci of tumor cells with osteoid formation were scattered throughout the lung and were accompanied by pulmonary emphysema and congestion. The hepatocytes showed diffuse fatty degeneration and sinusoids were dilated and congested. Tumor cells were not present in the liver. Immunohistochemistry revealed that tumor cells were negative with regard to anticytokeratin antibody but immunopositive with regard to antivimentin and antiosteocalcin antibodies (Figure 4). Osteocalcin antibody strongly reacted with the cytoplasm of the neoplastic cells, including giant cells.
Figure 2.
Numerous osteoclast-like giant cells (arrow) were present in the tumor tissue. Hematoxylin and eosin stain; bar, 20 μm.
Figure 3.
Abundant osteoid formation in the tumor tissue. Hematoxylin and eosin stain; bar, 20 μm.
Figure 4.
Tumor cells stained positively with antiosteocalcin monoclonal antibody. Bar, 10 μm.
Discussion
Canine osteosarcoma develops in large breeds, and body weight gain, particularly an increase in body height, constitutes the highest risk factor for osteosarcoma.16 The present rabbit was of average size. Although large species of rabbit exist, the correlation between the development of osteosarcoma and body size remains unclear. In dogs and cats, the appendicular skeleton is affected most frequently.16 About 75% of canine osteosarcomas arise in limb bones, about 2 times more frequently in the forelimbs than hindlimbs.8 In cats, osteosarcomas occur nearly 2 times more frequently in the hindlimbs than forelimbs.17 In contrast, half of the published rabbit cases of osteosarcoma developed in the skull or facial bones (Table 1). The next most common site in rabbits was an appendicular joint, as in the current case.
Table 1.
Osteosarcoma in rabbits
| Signalment (breed, age, sex) | Primary site | Metastasis | Histologic type | Reference |
| Dutch, 7.5 y, female | Skull | Present | Productive osteoblastic osteosarcoma | 1 |
| American chinchilla, 2.5 y, female | Multicentric; facial bone | Present; subcutaneous involvement | Productive osteoblastic osteosarcoma | 5 |
| Polish dwarf, 1 y, female | Sacrococcygeal joint | Present; subcutaneous involvement | Productive osteoblastic osteosarcoma | 11 |
| Not indicated, 7.5 y, female | Mandible | Not indicated | Not indicated | 6 |
| Rex, 7.5 y, male | Upper lip; extraskeletal | Not present | Fibroblastic osteosarcoma | 13 |
| Rex, 7.5 y, male | Rib | Present | Productive osteoblastic osteosarcoma | 14 |
| New Zealand white, 1.5 y, male | Mandible | Present | Productive osteoblastic osteosarcoma | 18 |
| New Zealand white, 6 y, male | Mandible | Present | Productive osteoblastic osteosarcoma | 20 |
| Not indicated, older than 2 y, female | Proximal tibia | Not present | Osteochondoroma? | 19 |
| Crossbred, 6 y, male | Glenohumeral joint | Not indicated | Productive osteoblastic osteosarcoma | 9 |
| Crossbred, 7 y, female | Intertarsal joint | Present | Productive osteoblastic osteosarcoma | Current |
Pulmonary metastases occur in most dogs with osteosarcoma of the limbs.15 Except for fibroblastic lesions, most rabbit osteosarcomas metastasize to the lungs, abdominal organs, or subcutaneous tissue (Table 1). The present rabbit had pulmonary micrometastases, suggesting that osteosarcoma in this species carries a high risk of metastasis. Canine osteosarcomas are considered to invade the joint cavity or juxta-articular bones only rarely.16 In the current rabbit, the tumor affected the tarsal joint region involving the distal tibia of the right hindlimb. It may be difficult from the clinical signalment to determine whether an osteosarcoma of the skeleton involved the joint or whether the joint itself was the primary site.
Osteosarcomas in dogs are classified histologically into poorly differentiated, osteoblastic, chondroblastic, fibroblastic, telangiectatic, and giant cell types.15,16 Some feline osteosarcomas are characterized by the presence of multinucleated giant cells, sometimes in large numbers.12 In the present case, the tumor was composed of ovoid to short spindle-shaped, histiocyte-like cells with abundant osteoid formation. In addition, the rabbit tumor contained many multinucleated giant cells, similar to a characteristic feature of feline osteosarcomas. Most previously reported cases of rabbit osteosarcoma have been classified as productive osteoblastic osteosarcoma, typically composed predominantly of osteoid and bone. Only one case of extraskeletal osteosarcoma in rabbits was reported to be of the fibroblastic type, with rudimentary osteoid formation.13
Osteocalcin is an osteoblast-specific marker. In an immunohistochemical study using antiosteocalcin antibody, osteocalcin had 70% sensitivity and 100% specificity for the detection of osteoblasts. In the present case, like the osteosarcoma in the right glenohumeral joint, isolated tumor cells consistently reacted with antiosteocalcin antibody.9 These results indicate the osteocalcin is a good choice for a diagnostic marker of the rabbit osteosarcoma
Serum ALP levels are often elevated in dogs with osteosarcoma.16 The serum ALP level was reported as an important prognostic factor in 75 dogs with osteosarcoma of the limbs.3 Serum ALP levels were higher in experimental rabbits with osteosarcoma than in normal rabbits.20 The serum ALP level in the present rabbit (147 U/L) was much higher than the normal range in rabbits (4 to 16 U/L).10 Because the current rabbit died soon after surgery, the correlation between the serum ALP level and prognosis remain unclear. However, evaluation of the ALP level is useful as a diagnostic factor for rabbits with osteosarcoma.
Although primary bone neoplasia may be uncommon in rabbits, several case reports have accumulated and facilitate the study of the clinical and pathologic features of osteosarcomas in this species.
References
- 1.Amand WB, Riser WH, Biery DN. 1973. Spontaneous osteosarcoma with widespread metastasis in a belted Dutch rabbit. J Am Anim Hosp Assoc 9:577–581 [Google Scholar]
- 2.Brodey RS, Riser WH. 1969. Canine osteosarcoma. A clinicopathologic study of 194 cases. Clin Orthop Relat Res 62:54–64 [PubMed] [Google Scholar]
- 3.Ehrhart N, Dernell WS, Hoffmann WE, Weigel RM, Powers BE, Withrow SJ. 1998. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990–1996). J Am Vet Med Assoc 213:1002–1006 [PubMed] [Google Scholar]
- 4.Fanburg JC, Rosenberg AE, Weaver DL, Leslie KO, Mann KG, Taatjes DJ, Tracy RP. 1997. Osteocalcin and osteonectin immunoreactivity in the diagnosis of osteosarcoma. Am J Clin Pathol 108:464–473 [DOI] [PubMed] [Google Scholar]
- 5.Hoover JP, Paulsen DB, Quallis CW, Bahr RJ. 1986. Osteogenic sarcoma with subcutaneous involvement in a rabbit. J Am Vet Med Assoc 189:1156–1158 [PubMed] [Google Scholar]
- 6.Jacobson SA. 1971. Comparative pathology of the tumors of bone. Springfield (IL): Charles C Thomas. [Google Scholar]
- 7.Klein MJ, Siegal GP. 2006. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol 125:555–581 [DOI] [PubMed] [Google Scholar]
- 8.Knecht CD, Priester WA. 1978. Musculoskeletal tumors in dogs. J Am Vet Med Assoc 172:72–74 [PubMed] [Google Scholar]
- 9.Kondo H, Ishikawa M, Maeda H, Onuma M, Masuda M, Shibuya H, Koie H, Sato T. 2007. Spontaneous osteosarcoma in a rabbit (Oryctolagus cuniculus). Vet Pathol 44:691–694 [DOI] [PubMed] [Google Scholar]
- 10.Mader DR. 2003. Basic approach to veterinary care, p 147–154. In: Quesenberry K, Carpenter JW. Ferrets, rabbits, and rodents: clinical medicine and surgery, 2nd ed. Philadelphia (PA): Saunders. [Google Scholar]
- 11.Mazzullo G, Russo M, Niutta PP, De Vico G. 2004. Osteosarcoma with multiple metastases and subcutaneous involvement in a rabbit (Oryctolagus cuniculus). Vet Clin Pathol 33:102–104 [DOI] [PubMed] [Google Scholar]
- 12.Quigley PJ, Leedale AH. 1983. Tumors involving bone in the domestic cat: a review of 58 cases. Vet Pathol 20:670–686 [DOI] [PubMed] [Google Scholar]
- 13.Renfrew H, Rest JR, Holden AR. 2001. Extraskeletal fibroblastic osteosarcoma in a rabbit (Oryctolagus cuniculus). J Small Anim Pract 42:456–458 [DOI] [PubMed] [Google Scholar]
- 14.Salm R, Field J. 1965. Osteosarcoma in a rabbit. J Pathol Bacteriol 89:400–402 [DOI] [PubMed] [Google Scholar]
- 15.Slayter MV, Boosinger TR, Pool RR, Dammrich K, Misdorp W, Larsen S. 1994. Malignant tumors. In: Histological classification of bone and joint tumors of domestic animals. World Health Organization, 2nd series, vol 1. Washington (DC): Armed Forces Institute of Pathology. [Google Scholar]
- 16.Thompson KG, Pool RR. 2002. Tumors of bones, p 245–317. In: Meuten J. Tumors in domestic animals, 4th ed. Blackwell (IA): Iowa State Press. [Google Scholar]
- 17.Turrel JM, Pool RR. 1982. Primary bone tumors in the cat: a retrospective study of 15 cats and a literature review. Vet Radiol 23:152–166 [Google Scholar]
- 18.Walberg JA. 1981. Osteogenic sarcoma with metastasis in a rabbit (Oryctolagus cuniculus). Lab Anim Sci 31:407–408 [PubMed] [Google Scholar]
- 19.Weisbroth SH. 1972. Neoplastic diseases, p 331–375. In: Weisbroth SH, Flatt RE, Kraus AL. The biology of the laboratory rabbit. San Diego (CA): Academic Press. [Google Scholar]
- 20.Weisbroth SH, Hurvitz A. 1969. Spontaneous osteogenic sarcoma in Oryctolagus cuniculus with elevated serum alkaline phosphatase. Lab Anim Care 19:263–265 [PubMed] [Google Scholar]