Abstract
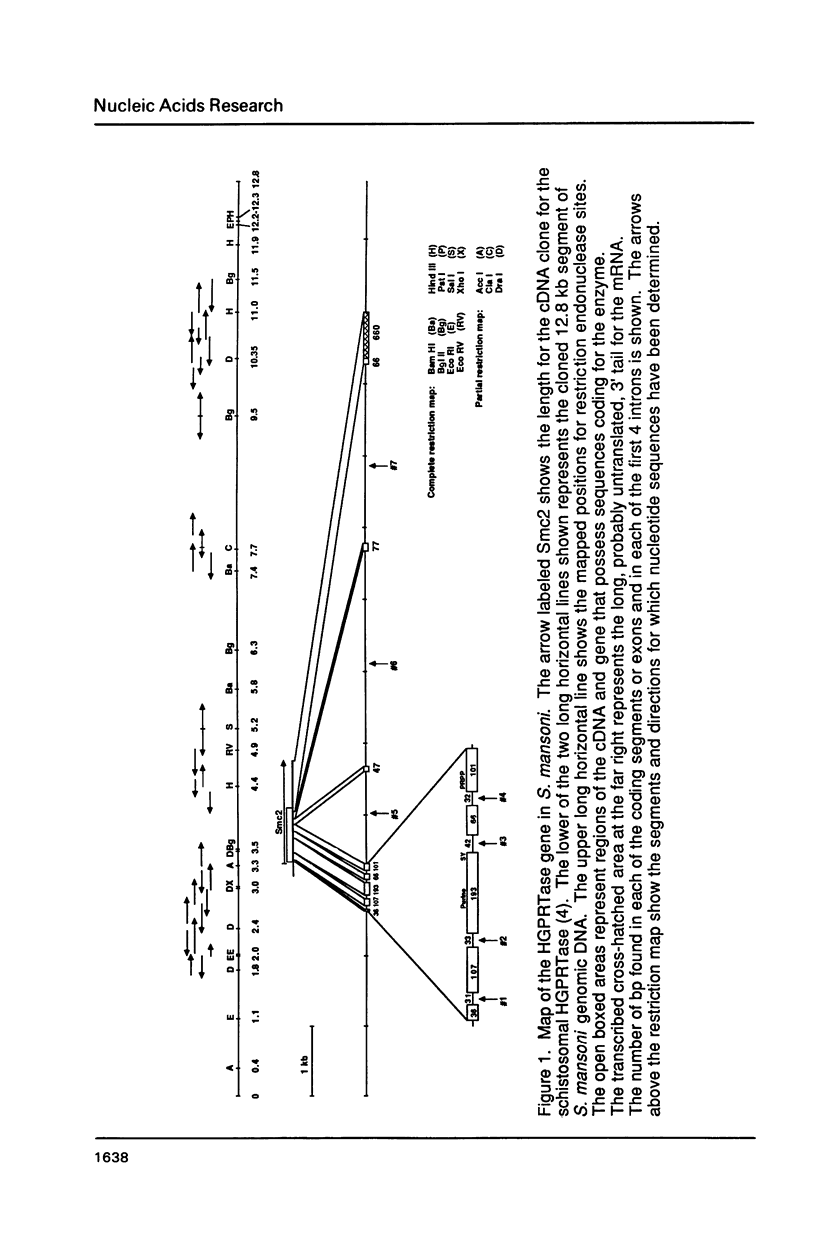
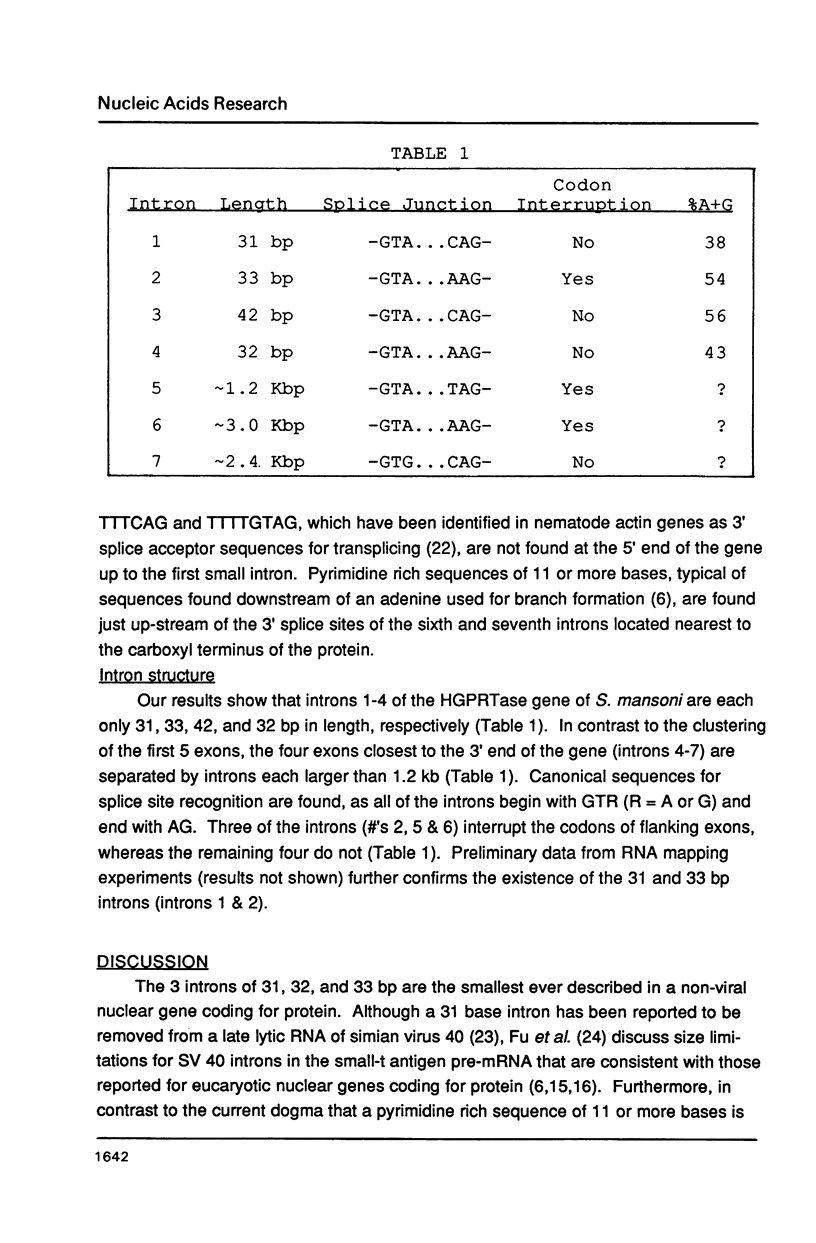
The single copy gene for the hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) of the parasitic trematode, Schistosoma mansoni, contains seven introns, the first four of which are only 31, 33, 42, and 32 bases in length. These are the smallest introns ever discovered in a non-viral nuclear gene coding for protein. These very small introns possess the canonical GT...AG splice site sequences but lack the branching sequence, the secondary structure, and the minimum size of approximately 50 bases believed to be required for the splicing of eucaryotic mRNA precursors. Evidently, a somewhat different splicing mechanism for the transcripts of these very small introns is necessary. Their discovery within the genes of helminths raises theoretical considerations for the evolution of introns in eucaryotes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A. Transcripts coding for variant surface glycoproteins of Trypanosoma brucei have a short, identical exon at their 5' end. Gene. 1982 Dec;20(2):281–289. doi: 10.1016/0378-1119(82)90046-4. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Craig S. P., 3rd, McKerrow J. H., Newport G. R., Wang C. C. Analysis of cDNA encoding the hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) of Schistosoma mansoni; a putative target for chemotherapy. Nucleic Acids Res. 1988 Jul 25;16(14B):7087–7101. doi: 10.1093/nar/16.14.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Sprang S., Fletterick R., Rutter W. J. Intron-exon splice junctions map at protein surfaces. Nature. 1982 Sep 9;299(5879):180–182. doi: 10.1038/299180a0. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Jeffries A. C., Sheldon C. C., Symons R. H. Structural and ionic requirements for self-cleavage of virusoid RNAs and trans self-cleavage of viroid RNA. Cold Spring Harb Symp Quant Biol. 1987;52:249–259. doi: 10.1101/sqb.1987.052.01.030. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Colgan J. D., Manley J. L. Multiple cis-acting sequence elements are required for efficient splicing of simian virus 40 small-t antigen pre-mRNA. Mol Cell Biol. 1988 Sep;8(9):3582–3590. doi: 10.1128/mcb.8.9.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Guyaux M., Cornelissen A. W., Pays E., Steinert M., Borst P. Trypanosoma brucei: a surface antigen mRNA is discontinuously transcribed from two distinct chromosomes. EMBO J. 1985 Apr;4(4):995–998. doi: 10.1002/j.1460-2075.1985.tb03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhiang S. M., Garey J. R., Riggs A. F. Exon-intron organization in genes of earthworm and vertebrate globins. Science. 1988 Apr 15;240(4850):334–336. doi: 10.1126/science.2832953. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. Protein structural domains in the Caenorhabditis elegans unc-54 myosin heavy chain gene are not separated by introns. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4253–4257. doi: 10.1073/pnas.80.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg N., Gilbert W. Intron/exon structure of the chicken pyruvate kinase gene. Cell. 1985 Jan;40(1):81–90. doi: 10.1016/0092-8674(85)90311-3. [DOI] [PubMed] [Google Scholar]
- Martinez H. M. An RNA secondary structure workbench. Nucleic Acids Res. 1988 Mar 11;16(5):1789–1798. doi: 10.1093/nar/16.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Gotoh Y. Signals for the selection of a splice site in pre-mRNA. Computer analysis of splice junction sequences and like sequences. J Mol Biol. 1987 May 20;195(2):247–259. doi: 10.1016/0022-2836(87)90647-4. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Watkins K. P., Agabian N. Trypanosome mRNAs share a common 5' spliced leader sequence. Cell. 1984 Aug;38(1):309–316. doi: 10.1016/0092-8674(84)90552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley F., Martin W. F., Cerff R. Intron conservation across the prokaryote-eukaryote boundary: structure of the nuclear gene for chloroplast glyceraldehyde-3-phosphate dehydrogenase from maize. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2672–2676. doi: 10.1073/pnas.85.8.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautmann G., Matthes H. W., Gait M. J., Breathnach R. Synthetic donor and acceptor splice sites function in an RNA polymerase B (II) transcription unit. EMBO J. 1984 Sep;3(9):2021–2028. doi: 10.1002/j.1460-2075.1984.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C. Accurate and efficient pre-mRNA splicing in Drosophila cell-free extracts. Proc Natl Acad Sci U S A. 1988 May;85(9):2904–2908. doi: 10.1073/pnas.85.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. Exon shuffling and intron insertion in serine protease genes. Nature. 1985 Jun 6;315(6019):458–459. doi: 10.1038/315458a0. [DOI] [PubMed] [Google Scholar]
- Russnak R. H., Candido E. P. Locus encoding a family of small heat shock genes in Caenorhabditis elegans: two genes duplicated to form a 3.8-kilobase inverted repeat. Mol Cell Biol. 1985 Jun;5(6):1268–1278. doi: 10.1128/mcb.5.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Konarksa M. M., Grabowski P. J., Lamond A. I., Marciniak R., Seiler S. R. Splicing of messenger RNA precursors. Cold Spring Harb Symp Quant Biol. 1987;52:277–285. doi: 10.1101/sqb.1987.052.01.033. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Trans splicing: variation on a familiar theme? Cell. 1987 Jul 17;50(2):147–148. doi: 10.1016/0092-8674(87)90207-8. [DOI] [PubMed] [Google Scholar]
- Spieth J., Denison K., Zucker E., Blumenthal T. The nucleotide sequence of a nematode vitellogenin gene. Nucleic Acids Res. 1985 Oct 11;13(19):7129–7138. doi: 10.1093/nar/13.19.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. D., Conrad R. C., Blumenthal T. The C. elegans trans-spliced leader RNA is bound to Sm and has a trimethylguanosine cap. Cell. 1988 Aug 12;54(4):533–539. doi: 10.1016/0092-8674(88)90075-x. [DOI] [PubMed] [Google Scholar]
- Thompson-Jäger S., Domdey H. Yeast pre-mRNA splicing requires a minimum distance between the 5' splice site and the internal branch acceptor site. Mol Cell Biol. 1987 Nov;7(11):4010–4016. doi: 10.1128/mcb.7.11.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Yarbrough P. O., Hayden M. A., Dunn L. A., Vermersch P. S., Klass M. R., Hecht R. M. The glyceraldehyde-3-phosphate dehydrogenase gene family in the nematode, Caenorhabditis elegans: isolation and characterization of one of the genes. Biochim Biophys Acta. 1987 Jan 28;908(1):21–33. doi: 10.1016/0167-4781(87)90018-2. [DOI] [PubMed] [Google Scholar]
- Zakut R., Shani M., Givol D., Neuman S., Yaffe D., Nudel U. Nucleotide sequence of the rat skeletal muscle actin gene. Nature. 1982 Aug 26;298(5877):857–859. doi: 10.1038/298857a0. [DOI] [PubMed] [Google Scholar]