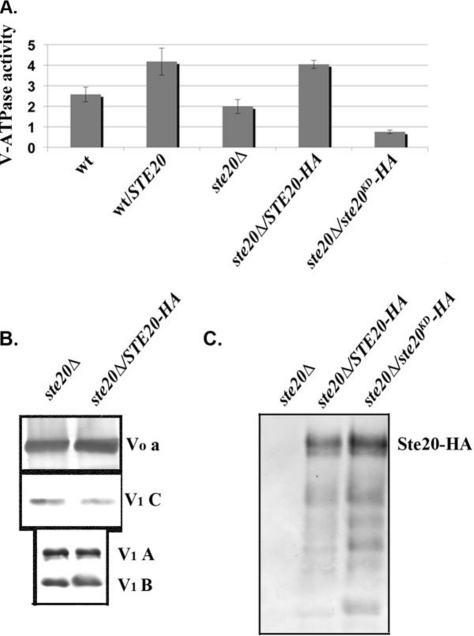
Fig 4.
Ste20 localizes to the vacuole and activates V-ATPase activity. (A and B) Vacuolar vesicles were isolated from wild-type cells, wild-type cells transformed with a multicopy plasmid expressing STE20 from its native promoter (wt/STE20), a ste20Δ mutant, and the ste20Δ mutant transformed with a multicopy plasmid bearing either 3HA-tagged STE20 (STE20-HA) or a 3HA-tagged kinase-dead mutant of STE20 (ste20KD-HA). (A) Concanamycin A-sensitive ATPase activity in the isolated vacuolar vesicles is expressed as μmol ATP hydrolyzed/min/mg vacuolar protein. For each sample, the mean ± standard error of the mean (SEM) for four to seven independent vacuolar preparations is shown. (B) Vacuolar vesicles from the ste20Δ and STE20-3HA-overexpressing cells were solubilized, and equivalent quantities of vacuolar protein were separated by SDS-PAGE and transferred to nitrocellulose, followed by immunoblotting with antibodies to subunit a of the V-ATPase V0 sector and subunits A, B, and C of the V-ATPase V1 sector. (C) Localization of wild-type and kinase-dead HA-tagged Ste20 to vacuolar membranes. Equal concentrations of vacuolar protein were separated by SDS-PAGE as in panel B and then probed for the presence of Ste20-HA on immunoblots. The predicted molecular mass of Ste20-3HA is indicated.