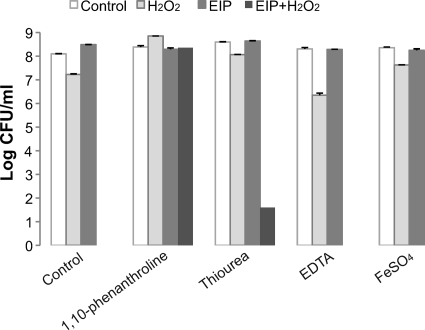
Fig 10.
Effects of ferrous ion chelators, hydroxyl radical scavengers, or ferrous ions on the bactericidal activity of EIP-K plus H2O2. Bacteria were pretreated with a ferrous chelator, 1,10-phenanthroline (100 μM), and hydroxyl radical scavengers, mannitol (100 μM) and thiourea (10 mM), for 30 min, followed by treatment with ddH2O (control), 13.75 mM EIP-K, 3 mM H2O2, or 13.75 mM EIP-K plus 3 mM H2O2. EDTA (100 μM) and FeSO4 (10 μM) were presented at the same time as the treatment with escapin products. The values are means and SEM for two experiments, each run in duplicate. Two-way ANOVA shows a significant treatment effect (F[3,23] = 145.34; P = 0.0000001), a significant chelator effect (F[4,23] = 128.50; P = 0.0000001), and a significant treatment-chelator interaction effect (F[12,23] = 58.57; P = 0.0000001).