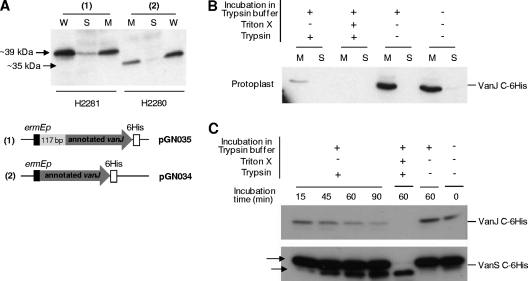
Fig 5.
(A) VanJ is predominantly localized in the membrane. Western immunoblot analysis indicates that the vast majority of His-tagged VanJ protein detectable in whole cells (W) subfractionates to the membrane fraction (M) and not the soluble cytoplasmic fraction (S). Both the short (annotated) and long (experimentally determined) forms of VanJ are membrane localized. (B) Protoplast-shaving experiments demonstrate that VanJ is exposed on the external face of the membrane. Protoplasts prepared from the H2281 strain expressing His-tagged VanJ were incubated with or without trypsin (as indicated) before being lysed and separated into membrane (M) and soluble cytoplasmic (S) fractions. Immunoblotting against the C-terminally located His tag indicates it is removed on exposure of the protoplasts to trypsin, consistent with external localization. (C) Time course protoplast-shaving experiments showing the gradual disappearance of His-tagged VanJ following treatment with trypsin. Experiments were performed as described for panel B and are compared to a similar analysis of His-tagged VanS (using strain H2276) as both a positive and a negative control. The arrows indicate both the appearance of a smaller N-terminally truncated form of VanS (positive control) and the net persistence of the two detectable forms of His-tagged VanS due to intracellular localization of the C-terminal tag (negative control).