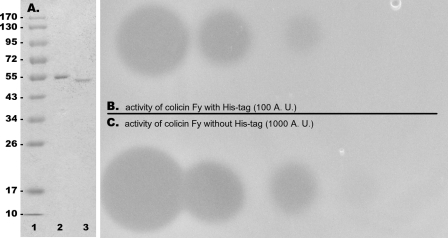
Fig 2.
Purification of colicin FY and its biological activity. (A) Purification of colicin FY containing an N-terminal histidine tag by using Ni and ion-exchange columns. Lane 1, low-molecular-weight protein standard (PageRuler Prestained Protein Ladder, Fermentas); lane 2, purified colicin FY with an N-terminal histidine tag; lane 3, purified colicin FY with an enterokinase-cleaved N-terminal histidine tag. A 12% polyacrylamide gel was stained with Coomassie brilliant blue. The values on the left are molecular sizes in kilodaltons. (B) Antibacterial activity of purified, His-tagged colicin FY on Y. kristensenii Y276 indicator strain. (C) Antibacterial activity of enterokinase-treated, purified colicin FY. Note that the biological activity of enterokinase-treated, purified colicin FY (with the N-terminal His tag removed) was increased approximately by an order of magnitude to 103 arbitrary units per μl. Based on the estimated number of colicin FY molecules (1012 in this purified sample) and the number of lethal colicin units in one arbitrary unit for colicins E1 to E9 (arbitrary unit = 2 × 108 lethal units [66]), one lethal unit (i.e., the lowest number of colicin molecules able to kill one susceptible bacterium) of colicin FY corresponds to approximately 5 molecules.