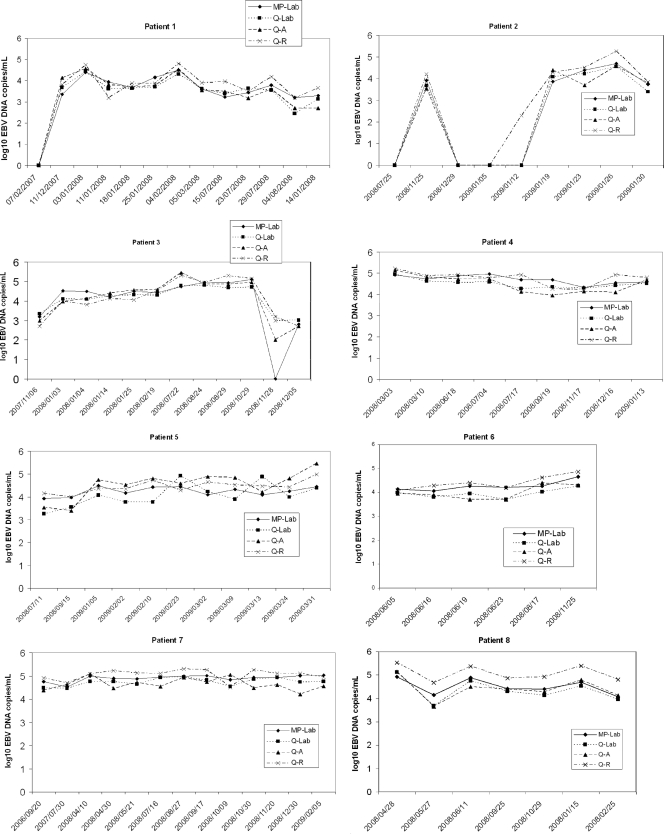
Fig 2.
Follow-up, using four different EBV DNA load technologies, for eight patients with various pathologies. Patient 1 was a hematopoietic stem cell transplantation recipient with non-Hodgkin lymphoma; patient 2, a hematopoietic stem cell transplantation recipient (donor EBV serological status, positive; recipient serological status, negative [D+ R−]); patients 3, 4, and 5, kidney transplant recipients (D+ R−). Patient 6 had HIV; patient 7, hypogammaglobulinemia with primary EBV infection; patient 8, EBV encephalitis. MP, MagNA Pure extraction; Q, QIAcube extraction; Lab, laboratory-developed real-time PCR (on a LightCycler amplification platform); A, Qiagen artus EBV RG PCR kit (on a Rotor-Gene amplification platform); R, Argene EBV R-gene kit (on a Rotor-Gene amplification platform).