Fig 6.
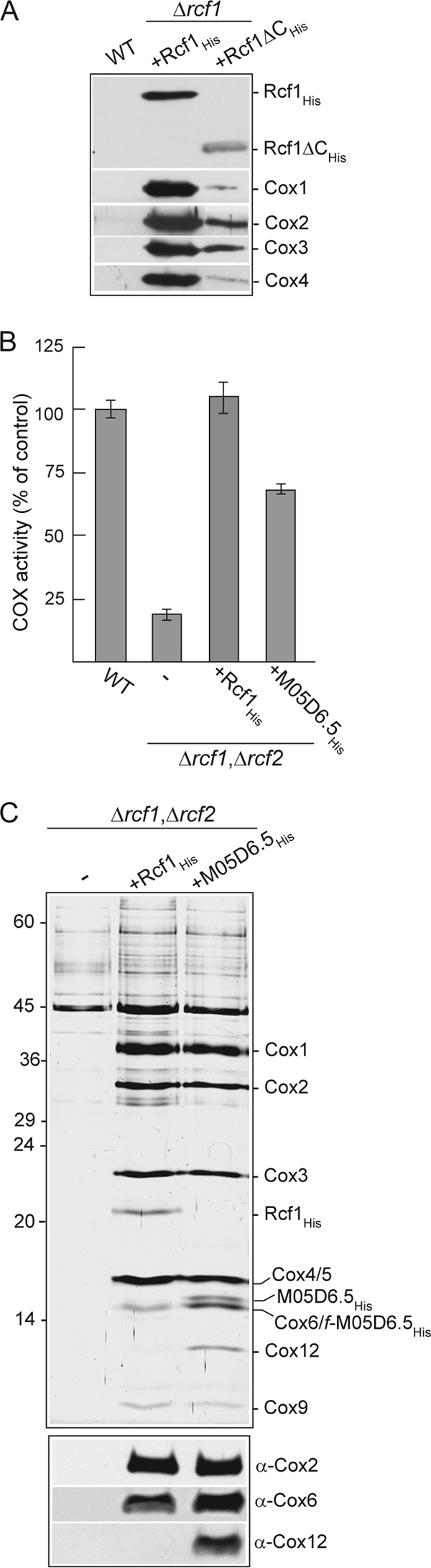
Physical association with and supporting activity of the COX enzyme are a conserved feature of Hig1 proteins. (A) Ni-NTA purification of Rcf1His and Rcf1ΔCHis following solubilization with Triton X-100. See Fig. 1B for further details. (B) COX enzyme activity measurements in mitochondria isolated from wild-type and Δrcf1 Δrcf2 strains harboring Rcf1His or the C. elegans Hig1 homolog M05D6.5His, as described for Fig. 3B. (C) Purification of Rcf1His and M05D6.5His from the Δrcf1 Δrcf2 strain performed under Triton X-100 solubilization conditions and analyzed by SDS-PAGE and silver staining (top) or in parallel by immunodecoration for Cox2, Cox6, and Cox12 subunits (bottom). The positions of COX subunits, Rcf1His, and M05D6.5His (and a smaller proteolytic fragment of this protein, f-M05D6.5His) on the silver-stained gel, verified by immunodecoration, are indicated.
