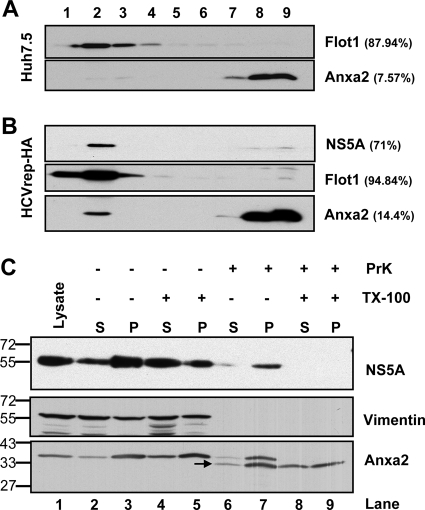
Fig 2.
Association of Anxa2 protein with detergent-resistant membrane fractions. (A) Huh7.5 naïve cells and (B) HCVrep-HA replicon cells were harvested at 24 h after seeding. Cell lysates were subjected to 1% Triton X-100 treatment and fractionated by discontinuous OptiPrep gradient centrifugation as described in Materials and Methods. Fractions were analyzed on a 10% SDS-PAGE gel, followed by immunoblotting with monoclonal antibodies against Anxa2, Flot1, and NS5A. Fractions are numbered from 1 to 9 in order from top to bottom (light to heavy). Results of Western blotting were quantified by PhosphorImager counting. The percentage of each protein in the DRM fraction (lanes 1 to 4) relative to the total signal intensity of the protein in all 9 fractions is indicated in parentheses. (C) Protection of HCV NSPs and Anxa2 by membranes against protease digestion. The postnuclear supernatant (PNS) from HCV-EV71I-Luc replicon cells were loaded as such (lane 1) or treated with 0.5% Triton X-100 (lanes 4 and 5 and lanes 8 and 9) or left untreated (lanes 2 and 3 and lanes 6 and 7), and this was followed by no digestion (lane 2 to 5) or treatment with proteinase K (lane 6 to 9). Thus, treated PNSs were fractionated into a 10,000 × g pellet (P) and supernatant (S) and analyzed for the presence of HCV NS5A, vimentin, and Anxa2. The positions of protein size markers are indicated on the left, and the arrow indicates the degraded form of Anxa2.