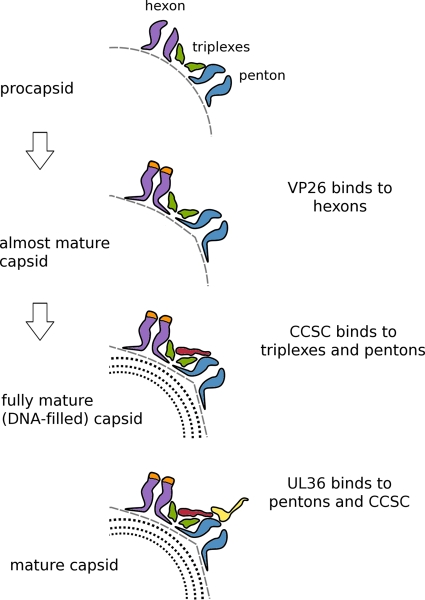
Fig 6.
Cartoon summarizing regulation of the sequential addition of proteins to the HSV-1 capsid. In the first step, as the procapsid undergoes initial maturation, its shape alters from round to polyhedral, and the hexon protrusions close up and become 6-fold symmetric, creating binding sites for VP26. Late in DNA packaging, another less extreme transition takes place that affects the peripentonal triplexes and/or the penton so as to enhance the affinity of this composite binding site for the CCSC, whose binding promotes nuclear exit. Subsequently, (11, 20), UL36 binds to another composite site involving both the CCSC and UL19 penton tips. In this way, UL25/UL17 plays a functional role in the cytoplasm similar to that which it plays in the nucleus, allowing only DNA-filled capsids to progress efficiently along the exit pathway.