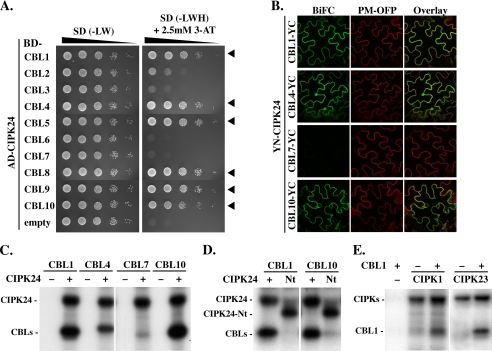
FIGURE 3.
CBL phosphorylation by CIPKs is a general feature of the CBL-CIPK network. A, 10-fold dilutions (A600 of 1–10−4) of the yeast PJ69-4A strain harboring an activation domain (AD)-CIPK24 and either empty DNA-binding domain (BD)-vector or different BD-CBL constructs were spotted on SD selection media without Leu and Trp (−LW; selection for positive transformants) or without Leu, Trp, and His (−LWH and 2.5 mm 3-amino-1,2,4-triazole; selection for interaction) and incubated for 8 days at 23 °C. Yeast two-hybrid assays indicate strong interaction of CIPK24 with CBL1, -4, -5, and -8–10 as indicated by arrowheads. B, BiFC analyses in transiently transformed N. benthamiana epidermal cells confirmed the interaction of YN-CIPK24 with CBL1-, CBL4-, and CBL10-YC but indicated no interaction with CBL7-YC. The plasma membrane marker (PM-OFP) (38) was co-expressed as a positive expression control. C, autoradiograph of in vitro phosphorylation assays in the absence (−) or presence (+) of CIPK24 with 50 ng of indicated CBL proteins. CIPK24 autophosphorylated and phosphorylated CBL1, CBL4, and CBL10 but not CBL7. D, only CIPK24 but not CIPK24-Nt (Nt) phosphorylated CBL1 (left) and CBL10 (right) in in vitro kinase assays. However, both kinase versions were able to autophosphorylate. E, in vitro kinase assays of CIPK1 (left) and CIPK23 (right). Both kinases phosphorylated CBL1 and exhibited autophosphorylation activity in presence (+) and in absence (−) of CBL1.