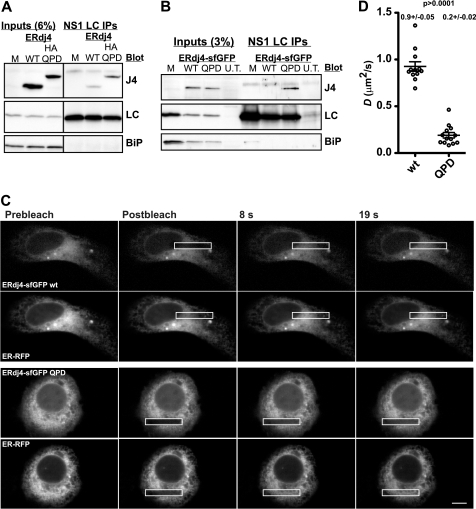
FIGURE 3.
Mutant ERdj4sfGFP is significantly less mobile. Mutant ERdj4 binds with high affinity to substrates as revealed in co-immunoprecipitation of ERdj4 and substrate experiments (A and B). A, COS-1 cells were transfected with ERdj4 or an HA-tagged ERdj4 QPD (H54Q) mutant in addition to NS1 κ LC and BiP and harvested at 24 h. The cells not transfected with ERdj4 (M), and untransfected cells (U.T.) were used as controls. Cell lysates were immunoprecipitated with anti-κ light chain, subjected to electrophoresis, and immunoblotted with the indicated antibodies. B, conditions were identical as in A, except cells were transfected with the indicated ERdj4-sfGFP variants. In both A and B, minimal amounts of WTERdj4 are detected, whereas substantial levels of mutant associate with LC. C, FRAP images series of HeLa cells co-expressing WT or QPD ERdj4-sfGFP and ER-RFP. Note the poor recovery of QPD ERdj4-sfGFP. D, plot of D values WT and QPD ERdj4-sfGFP expressed in U2OS cells and analyzed by FRAP.