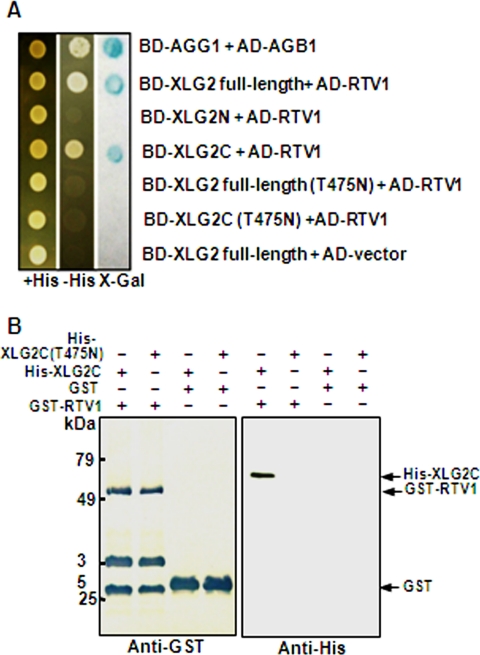
FIGURE 3.
XLG2 but not XLG2(T475N) interacts with RTV1. A, yeast two-hybrid assay. β-Galactosidase activity was measured by the filter assay method (Invitrogen). The domains within XLG2 responsible for RTV1 binding were investigated. Wild type, domain deletion mutants, and a point mutant of XLG2 (T475N) were fused to the bait (BD) vector, and a full-length cDNA of RTV1 was fused to the prey (AD) vector. These constructs (described at the right) were co-transformed into pJG69-4A yeast cells, and their interactions were evaluated. AGG1 and AGB1 were used as a positive control for interaction (14). B, in vitro protein pull-down assay. Approximately 1 μg of GST or recombinant GST-tagged RTV1 was incubated with 1 μg of recombinant His-tagged AtXLG2C or AtXLG2C(T475N) and pulled down with GST resin after preblocking with 1% BSA. After five washes, the bound protein was fractionated by 12% SDS-PAGE and subjected to Western blot analysis with anti-GST or anti-His monoclonal antibodies. The extra bands may represent nonspecific cross-reactivity to bacterial proteins. Detection was by alkaline phosphatase/NBT directly on the blot membrane (left) or by enhanced chemiluminescence on x-ray film (right).