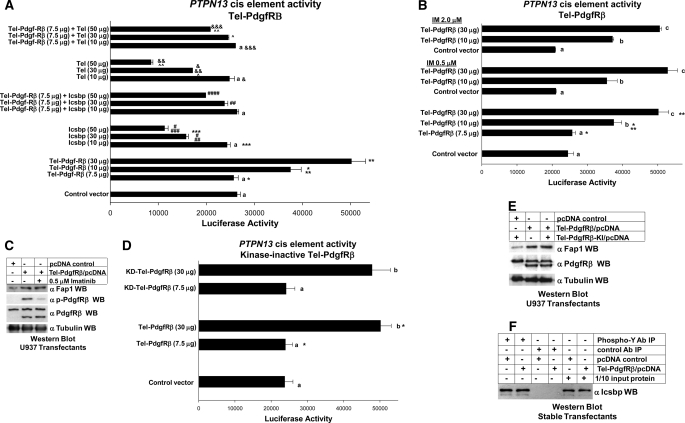
FIGURE 6.
Tel-PdgfRβ impairs repression of the Icsbp-binding PTPN13 by Icsbp and Tel. A, Tel-PdgfRβ expression increased activity of the PTPN13 cis element and impaired repression by overexpressed Icsbp and Tel. U937 cells were stably transfected with a vector to express Tel-PdgfR (or control expression vector) and co-transfected with a reporter vector with three copies of the Icsbp-binding PTPN13 cis element linked to a minimal promoter (ptpn13L3-p) or control reporter vector, and vectors to express various amounts of Icsbp or Tel (or empty control vector). Activity from the empty reporter vector was <10% of the ptpn13GL3-p, which was subtracted as background. Reporter activity not statistically significant (p > 0.2) is indicated by a lowercase a. Statistically significant differences in reporter activity are indicated by *, **, ***, #, ##, ###, &, &&, &&&, ∧, or ∧∧. B, Tel-PdgfRβ-induced PTPN13 cis element activity is independent of tyrosine kinase activity of the fusion protein (imatinib studies). U937 cells that were stably transfected with a vector to express Tel-PdgfR or control expression vector were co-transfected with a reporter vector with three copies of the Icsbp-binding PTPN13 cis element linked to a minimal promoter (ptpn13GL3-p) or control reporter vector. Some transfectants were treated with the tyrosine kinase inhibitor imatinib (IM). Activity from the empty reporter vector was <10% of the ptpn13GL3-p, which was subtracted as background. Reporter not statistically significantly different (p > 0.2 for all comparisons) is indicated by a lowercase letter (a, b, and c). Statistically significant differences (p < 0.01 for all comparisons) in reporter activity are indicated by * or **. C, Tel-PdgfRβ-induced increase in Fap1 protein expression is not inhibited by imatinib. U937 myeloid cells were stably transfected with a vector to express Tel-PdgfRβ or empty control vector. Some transfectants were treated with imatinib as indicated. Total cell lysates were analyzed for Tel-PdgfRβ tyrosine phosphorylation (activation) by Western blots (WB), which were serially probed with antibodies to Fap1, phospho-PdgfRβ, total PdgfRβ, or tubulin (as a loading control). This blot represents transfectants performed at the same time as one of the reporter gene assays from B. D, Tel-PdgfRβ-induced PTPN13 cis element activity is independent of tyrosine kinase activity of the fusion protein (studies with kinase inactive fusion protein). U937 cells that were stably transfected with a vector to express Tel-PdgfRβ, a kinase inactive form of Tel-PdgfRβ (KD-Tel-PdgfRβ), or control expression vector were co-transfected with a reporter vector with three copies of the Icsbp-binding PTPN13 cis element linked to a minimal promoter (ptpn13GL3-p) or control reporter vector. Reporter activity not statistically significantly different is indicated by a lowercase letter (a, b, and c). Statistically significant differences in reporter activity are indicated by *. E, Tel-PdgfRβ increased Fap1 protein expression in a tyrosine kinase independent manner. U937 myeloid cells were stably transfected with a vector to express Tel-PdgfRβ, KD-Tel-PdgfRβ, or empty control vector. Total cell lysates were analyzed by Western blots, which were serially probed with antibodies to PdgfRβ, Fap1, or tubulin (as a loading control). The anti-PdgfRβ antibodies used in this experiment recognize both endogenous PdgfRβ and the product of the Tel-PdgfRβ transgene. This blot represents transfectants performed at the same time as one of the reporter gene assays from B. F, Tel-PdgfRβ expression does not alter expression or tyrosine phosphorylation of Icsbp in myeloid cell line transfectants. Tel-PdgfRβ stable transfectants were also analyzed for phosphorylation and expression of Icsbp. Cell lysates were immunoprecipitated (under denaturing conditions) with an antibody to phosphotyrosine or irrelevant control antibody. Co-precipitating proteins were separated by SDS-PAGE and Western blots were probed with an antibody to Icsbp. Nonprecipitated lysates were also included on the SDS-PAGE (indicated as 1/10 input protein) as a loading control for Icsbp abundance.