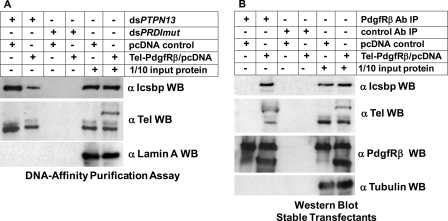
FIGURE 8.
Tel-PdgfRβ interacted with Tel and Icsbp and decreased binding of these proteins to the PTPN13 cis element. A, Tel-PdgfRβ expression in myeloid cell lines decreased binding of Tel and Icsbp to the PTPN13 cis element in vitro. DNA affinity purification assays were performed with KG1 nuclear proteins stably expressing Tel-PdgfRβ (or with empty control vector), and biotin-labeled, double-stranded oligonucleotide probes representing the Icsbp/Tel binding site in the PTPN13 promoter (dsPTPN13) or a non-Icsbp binding mutant sequence (dsPRDImut). Affinity purified proteins were separated by SDS-PAGE and Western blots were probed with antibodies to Icsbp and Tel (using an antibody that recognizes both wild type Tel and the Tel-PdgfRβ fusion protein). Nonprecipitated proteins were also included on the SDS-PAGE (indicated as 1/10 input protein) and Western blots were probed with a Lamin A antibody as a loading control. B, Tel and Icsbp co-immunoprecipitated with Tel-PdgfRβ from myeloid cells. Total cell lysates from these KG1 stable transfectants were immunoprecipitated with an antibody to PdgfRβ (or with irrelevant antibody control). Immunoprecipitates were separated by SDS-PAGE and Western blots were serially probed with antibodies to Icsbp, Tel, or PdgfRβ. The Tel and PdgfRβ antibodies used in these experiments recognize the endogenous protein and the Tel-PdgfRβ fusion proteins. Nonprecipitated proteins were included on the SDS-PAGE (indicated as 1/10 input protein) and Western blots were also probed with tubulin antibody as a loading control.