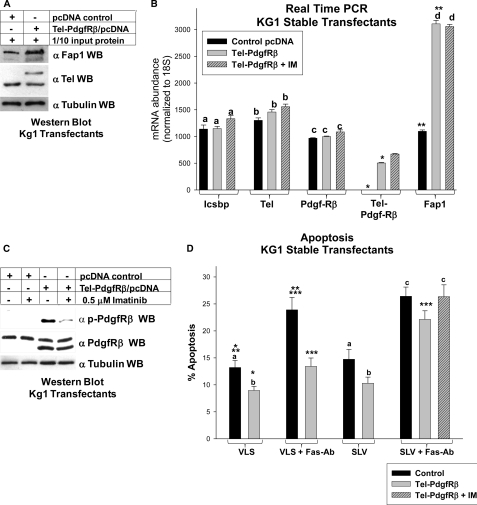
FIGURE 9.
Tel-PdgfRβ increased Fap1 expression and induced Fas resistance in myeloid cells. A, Tel-PdgfRβ increased Fap1 protein expression in KG1 myeloid cells. KG1 cells were stably transfected with a vector to express Tel-PdgfRβ or control vector and analyzed for Fap1 protein expression. Western blots of total cell lysates were serially probed with antibodies to Fap1, Tel (using an antibody which identifies endogenous Tel and Tel-PdgfRβ), or tubulin (as a loading control). C, Tel-PdgfRβ increased Fap1 mRNA expression in KG1 myeloid cells. The KG1 stable transfectants described above were also analyzed for Fap1 mRNA expression by real time PCR. Expression of Icsbp, endogenous Tel, endogenous PdgfRβ, and Tel-PdgfRβ were also determined. For these experiments, Tel- and PdgfRβ-specific primer sets were used, which do not recognize the Tel-PdgfRβ fusion transcript, and a Tel-PdgfRβ primer set was used, which only recognizes the fusion transcript. Nonstatistically significant differences in mRNA expression (p > 0.05 for all comparisons) are indicated by lowercase letters (a, b, c, and d). Statistically significant differences (p < 0.001 for all comparisons) in expression are indicated by * or **. B, treatment with imatinib impaired Tel-PdgfRβ autoactivation in KG1 transfectants. KG1 myeloid cells were stably transfected with a vector to express the Tel-PdgfRβ fusion protein or empty control vector. Untreated cells were compared with cells treated with imatinib (IM) as indicated. Total cell lysates were analyzed for Tel-PdgfRβ tyrosine phosphorylation (activation) by Western blots, which were serially probed with antibodies to phospho-PdgfRβ, total PdgfRβ, or tubulin (as a loading control). The anti-PdgfRβ antibodies used in this experiment recognize both endogenous PdgfRβ and the product of the Tel-PdgfRβ transgene. C, Tel-PdgfRβ induced Fap1-dependent Fas resistance in KG1 myeloid cells. KG1 stable transfectants with Tel-PdgfRβ or control vector were analyzed for Fas-induced apoptosis. Cells were treated with SLV (Fas/Fap1 blocking peptide) or VLS control peptide, and Fas-agonist antibody or irrelevant control antibody. Some cells were additionally treated with imatinib. Apoptosis was determined by annexin V staining and flow cytometry, and expressed as % apoptotic cells. Differences in apoptosis not statistically significantly different (p > 0.05 for all comparisons) are indicated by lowercase letters (a, b, and c). Statistically significant differences (p < 0.001 for all comparisons) are indicated by *, **, or ***.