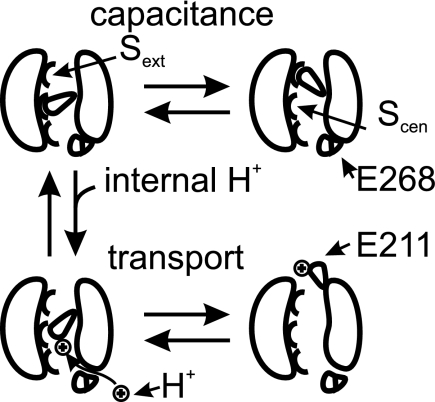
FIGURE 7.
Hypothetical model representing protonation of gating glutamate at central anion-binding site. When the gating glutamate 211 is in its negative unprotonated form, it can swing between the central (Scen) and external (Sext) anion-binding sites. At these positions, the gating glutamate 211 occludes the anion permeation pathway, and ClC-5 is not mediating ion transport. The motion is associated with the changes in nonlinear capacitance observed in ClC-5. Protonation via a pathway including the proton glutamate 268 allows reallocation of the gating glutamate 211 toward the extracellular lumen and transport. No capacitance changes are observed upon depolarization in this case. Internal pH and mutations of the proton glutamate 268 will modulate the distribution between transporting and “capacitive” ClC-5.