Figure 1.
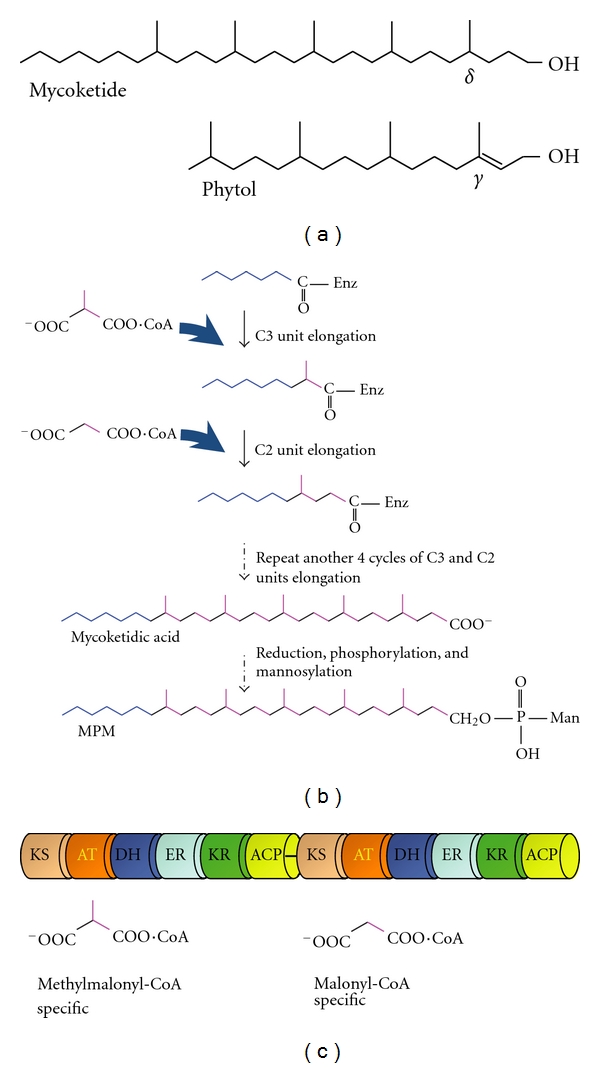
Biosynthesis of MPM. (a) Structures of mycoketide and phytol. Note that the first methyl branch of mycoketide is positioned at the δ-carbon whereas that of phytol, an isoprenoid compound, is located at the γ-carbon of phytol. (b) A predicted biosynthetic pathway of MPM. The chain elongation of mycoketide occurs on the Pks12 enzyme, followed by its release mediated by a yet unidentified hydrolase enzyme. (c) Schematic structure of Pks12 enzyme. Pks12 contains two tandemly aligned sets of catalytic domains (KS: ketosynthase; AT: acyltransferase; DH: dehydrogenase; ER: enoyl reductase; KR: ketoreductase; ACP: acyl carrier protein). The first set functions for a C3 unit elongation using methylmalonyl-CoA as a substrate and the second set for a C2 unit elongation using malonyl-CoA, which is controlled by the substrate specificities of the AT domains. Note that, unlike FAS enzymes, Pks12 lacks thioesterase domains.
