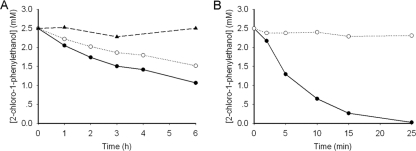
Fig 3.
Progress curves of the dehalogenation of 5 mM rac-2-CPE in 20 ml Tris-SO4 (200 mM, pH 8.0) at 30°C. Five-milligram crude extracts of each enzyme were used. (A) Reactions were carried out with wild-type HheA. Enantiomers of 2-CPE are shown as “●” for S and “○” for R. Chemical background was performed in the absence of enzyme and is indicated as “▲”. (B) The reaction was catalyzed by the N178A variant. Enantiomers of 2-CPE are shown as “●” for S and “○” for R.