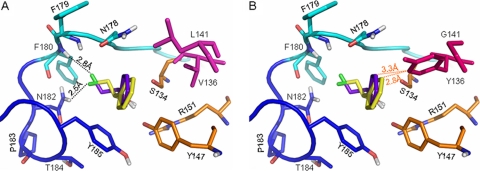
Fig 4.
Representation of the binding modes of the two enantiomers of 2-CPE in the active site of wild-type HheA (A) or the V136Y/L141G variant (B). The catalytic triad Ser134/Tyr147/Arg151 is shown in orange; V136 and L141 are in magenta, while Y136 and G141 are in hot pink; the 177Pro-Asn-Phe-Phe180 part of the halide-binding loop is in cyan, while the 181Asn-Asn-Pro-Thr-Tyr-185 part is in blue; the loop residues are shown in the corresponding cyan and blue, respectively. The docked substrates are shown in purple-blue for the R enantiomer and yellow for its S counterpart. Hydrogen bonds are indicated as black dotted lines. For clarity, van der Waals contacts are shown as orange dotted lines.