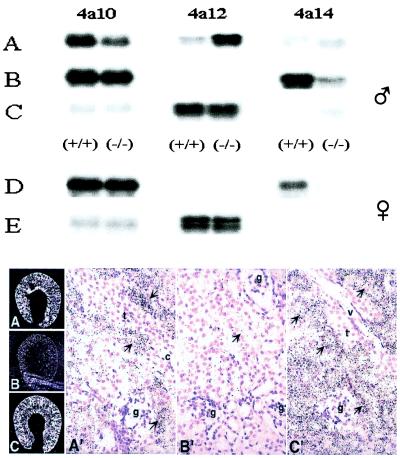
Figure 4.
Nucleic acid and in situ hybridization analysis of RNAs present in kidneys of control and DHT-treated adult mice. Top frame: Samples of total RNA (5–10 μg each) from the kidneys of control (A), castrated (B), castrated and DHT-treated (C) males or from control (D) and DHT-treated (E) females were fractionated by agar electrophoresis, transferred to nitrocellulose membranes, and hybridized to 32P-labeled DNA probes (400–500 bp) coding for segments of the 3′-untranslated end of the Cyp 4a10, 4a12, and 4a14 cDNAs. After high-stringency washes, the membranes were exposed to x-ray films for 4, 2, or 21 h for male Cyp 4a10, 4a12, and 4a14, respectively, and 6, 21, or 12 h for female Cyp 4a10, 4a12, and 4a14, respectively. RNA loadings were normalized by using a β-actin cDNA probe. Animal treatment protocols were as in Fig. 2 and Table 2. Long exposures revealed the presence of Cyp 4a14 reactive transcripts in 4a14 (−/−) mice kidneys (for example, lanes A–C). Reverse transcription–PCR amplification, cDNA cloning, and sequence analysis demonstrated that these were truncated mRNAs lacking exons × and XI, transcribed from the disrupted Cyp 4a14 gene. Bottom frame: Dehydrated paraffin sections from the kidneys of control (A and A′), castrated (B and B′) and DHT-treated castrated male mice (C and C′) were hybridized to [35S]-labeled riboprobes encoding 3′-end untranslated segments of the Cyp 4a12 cDNA. After washing, RNase A treatment, and dehydration, the sections were dipped in emulsion (IlfordK5; Knutsford, Cheshire, U.K.), exposed for 4–5 days at 4°C, and developed by using D-19 (Kodak). Slides were counterstained with hematoxylin. Photomicrographs were obtained by using either dark-field (3×) (A–C) or bright-field (100×) (A′, B′, and C′) optics. Thick ascending limbs, collecting ducts, glomeruli, and vessels (v) are indicated by arrows t, c, g, and v, respectively.