Abstract
Objective
To examine whether childhood traumatic stress increased the risk of developing autoimmune diseases as an adult.
Methods
Retrospective cohort study of 15,357 adult health maintenance organization members enrolled in the Adverse Childhood Experiences (ACEs) Study from 1995 to 1997 in San Diego, California, and eligible for follow-up through 2005. ACEs included childhood physical, emotional, or sexual abuse; witnessing domestic violence; growing up with household substance abuse, mental illness, parental divorce, and/or an incarcerated household member. The total number of ACEs (ACE Score range = 0–8) was used as a measure of cumulative childhood stress. The outcome was hospitalizations for any of 21 selected autoimmune diseases and 4 immunopathology groupings: T- helper 1 (Th1) (e.g., idiopathic myocarditis); T-helper 2 (Th2) (e.g., myasthenia gravis); Th2 rheumatic (e.g., rheumatoid arthritis); and mixed Th1/Th2 (e.g., autoimmune hemolytic anemia).
Results
Sixty-four percent reported at least one ACE. The event rate (per 10,000 person-years) for a first hospitalization with any autoimmune disease was 31.4 in women and 34.4 in men. First hospitalizations for any autoimmune disease increased with increasing number of ACEs (p < .05). Compared with persons with no ACEs, persons with ≥2 ACEs were at a 70% increased risk for hospitalizations with Th1, 80% increased risk for Th2, and 100% increased risk for rheumatic diseases (p < .05).
Conclusions
Childhood traumatic stress increased the likelihood of hospitalization with a diagnosed autoimmune disease decades into adulthood. These findings are consistent with recent biological studies on the impact of early life stress on subsequent inflammatory responses.
Keywords: childhood abuse, traumatic stress, autoimmune diseases, stress, inflammatory response
INTRODUCTION
Autoimmune diseases (ADs) are a heterogeneous group of 70 to 80 different inflammatory disorders affecting approximately 3% to 8% of the population in the United States (1). The prevalence of AD (14.7 to 23.5 million people) is reported to be steadily increasing (1,2). ADs occur when the immune response damages tissues in the body; ADs are classified according to the organ, tissue, or system targeted by the immune response. Disease may present as early as the second decade of life, but typically peaks in the third to sixth decade (3). Infectious agents and environmental factors are most commonly cited as etiologic factors (4,5). Because many ADs begin at a relatively young age and are chronic and treatments are palliative (2), they represent a significant personal and economic burden to individuals and their families.
AD immune response has been classified as T-helper 1 (Th1), T-helper 17 (Th17), or T-helper 2 (Th2) depending on the release of interferon (IFN)-γ, interleukin (IL)-17, or IL-4 from CD4+ T cells. However, both Th1 and Th2 ADs involve cell-mediated and antibody-mediated responses (5,6). ADs can be grouped according to the predominant immune mechanisms as follows: Th1, mixed Th1/Th2, and Th2; AD classically thought of as “rheumatoid” are classified as a subgroup of Th2 diseases. The risk for ADs may increase with age due to an increasing Th2 response and increasing numbers of autoantibodies (7).
Conservative estimates indicate that approximately 80% of the individuals with AD are women (2) because the basic immune response differs between men and women. For example, women respond to infection, immunization, or trauma with higher antibody production whereas inflammation is usually more severe in men (6,8–11). Sex differences in AD are also likely to be linked to sex-specific differences in glucocorticoid responses to stress because glucocorticoids decrease cell-mediated Th1-type immunity in response to acute stress (7). Estrogen transcriptionally upregulates glucocorticoid levels in females whereas testosterone decreases glucocorticoid levels in males (7,12–13).
The long-term health effects of childhood traumatic stress are well documented. For example, childhood abuse, neglect, and related forms of household dysfunction increase the risk of substance abuse, mental illness, sexually transmitted diseases, suicide attempts, and other health outcomes, such as ischemic heart disease (14–35). To date, there have been few studies that examine the contribution of childhood traumatic stress to the risk of developing an AD. A recent study by Danese and colleagues examined the association between childhood trauma and C-reactive protein (CRP), a biomarker of inflammation that may play a role in AD (36,37). After controlling for current stress, they reported that childhood maltreatment was associated with elevated CRP levels in adults 20 years later, suggesting that childhood maltreatment independently increases inflammation later in life (36).
Using longitudinal data from the Adverse Childhood Experiences (ACEs) Study, we created an ACE Score, using an integer count of eight interrelated and co-occurring (24,34,35) exposures of childhood adversity, to measure cumulative childhood traumatic stress. The relationship between the ACE Score and the risk of 21 different ADs as an adult was examined. We hypothesized that as the cumulative exposure to childhood stress and trauma increased the risk of developing AD in adulthood would also increase.
METHODS
Study Population
The ACE Study has been described elsewhere (14–35). More than 50,000 adult members of the Kaiser Foundation Health Plan in San Diego, California are evaluated annually at Kaiser Permanente's San Diego Health Appraisal Clinic (HAC). All Health Plan members who visit the HAC complete a standardized evaluation that included assessment of health history, health-related behaviors, a clinical review of systems, and psychosocial evaluations (14). Health plan members who completed the standardized HAC evaluation during 1995 to 1997 were eligible to participate in the ACE Study. They were mailed an ACE Study questionnaire with items about childhood exposure to abuse, neglect, domestic violence, and other related forms of serious household dysfunction (14). The survey was administered in two waves. Seventy percent (n = 9508) of adult members who were surveyed in Wave I (August through November 1995 and January through March 1996) and 65% (n = 8667) of adults who were surveyed in Wave 2 (June through October 1997) responded. Because 754 plan members inadvertently were mailed the survey twice, they were removed, resulting in a sample size of 17,421 at baseline. Of the 17,421 participants at baseline, 84 persons had incomplete information on race and educational attainment, leaving an analytic sample of 17,337 persons. The study was approved by the Institutional Review Boards of Kaiser Permanente and the Office for Protection from Research Risks at the National Institutes of Health.
The present analyses are based on follow-up medical record data available through December 31, 2005. Of the 17,337 participants included in prior analysis of the baseline data, 724 (4.2%) were excluded from the prospective phase of the study because the administrative enrollment database showed that their health maintenance organization (HMO) membership lapsed before their evaluation at the HAC or the member record number obtained from their clinic appointment was not considered valid. It is possible that persons might have disenrolled from the Kaiser Health Plan and then reenrolled back into the health plan during the follow-up period. To account for this occurrence, we calculated ratio of time disenrolled/total possible time enrolled. For persons who disenrolled and reenrolled at least once (median/mean = 1 time; range = 1–9 times) during the follow-up period, we excluded 1248 (7.2%) persons whose ratio of time disenrolled/total possible time enrolled was <80%. Although this “80% cut off” may be considered arbitrary, we considered such persons to have inadequate continuity of follow-up to merit consideration for inclusion in the prospective analysis. Thus, 15,365 of persons from the baseline sample were included in this analysis. An additional eight observations were deleted because their hospital admission date occurred before their baseline appointment date. Thus, the final sample size for analyses was 15,357 (88.6%).
ACEs
Questions used to define ACEs are listed in Table 1. All questions about ACEs pertained to the respondent's first 18 years of life (≤18 years of age). For questions adapted from the Conflict Tactics Scale (38), there were five response categories: (“never,” “once or twice,” “sometimes,” “often,” or “very often”). We defined the following three types of childhood abuse: emotional abuse (2 questions) (38), physical abuse (2 questions) (38), or contact sexual abuse (4 questions) (39). In addition, we defined five exposures to household dysfunction during childhood which included household exposure to substance abuse (defined by 2 questions) (40), mental illness (2 questions), violent treatment of female caretaker (4 questions) (38), criminal behavior in the household (1 question), and parental separation or divorce (1 question).
TABLE 1.
Definition and Baseline Prevalence of Categories of Adverse Childhood Experiences Reported by Adults Aged ≥19 Years by Sex: Adverse Childhood Experiences Study 1995 to 1997
| Adverse Childhood Experiences (ACEs) | Women, % (n = 8293) | Men, % (n = 7064) | Total, % (n = 15,357) |
|---|---|---|---|
| Emotional abuse | 12.8 | 7.4 | 10.3 |
| Did a parent or other adult in the household … | |||
| 1) Often or very often swear at you, insult you, or put you down? | |||
| 2) Sometimes, often, or very often act in a way that made you that you might be physically hurt? | |||
| Physical abuse | 26.7 | 29.7 | 28.0 |
| Did a parent or other adult in the household … | |||
| 1) Often or very often push, grab, slap, or throw something at you? | |||
| 2) Ever hit you so hard that you had marks or were injured? | |||
| Sexual abuse | 24.3 | 16.0 | 20.5 |
| Did an adult or person at least 5 years older ever … | |||
| 1) Touch or fondle you in a sexual way? | |||
| 2) Have you touch their body in a sexual way? | |||
| 3) Attempt oral, anal, or vaginal intercourse with you? | |||
| 4) Actually have oral, anal, or vaginal intercourse with you? | |||
| Substance abuse in household | 29.2 | 23.6 | 26.7 |
| 1) Live with anyone who was a problem drinker or alcoholic? | |||
| 2) Live with anyone who used street drugs? | |||
| Mental illness in household | 22.8 | 14.4 | 19.0 |
| 1) Was a household member depressed or mentally ill? | |||
| 2) Did a household member attempt suicide? | |||
| Mother treated violently | 13.5 | 11.5 | 12.6 |
| Was your mother or stepmother … | |||
| 1) Sometimes, often, or very often pushed, grabbed, slapped, or had something thrown at her? | |||
| 2) Sometimes, often, or very often kicked, bitten, hit with a fist, or hit with something hard? | |||
| 3) Ever repeatedly hit over at least a few minutes? | |||
| 4) Ever threatened with or hurt by a knife or gun? | |||
| Incarcerated household member | 5.0 | 3.9 | 4.5 |
| 1) Did a household member go to prison? | |||
| Parental separation or divorce | 23.8 | 21.6 | 22.8 |
| 1) Were your parents ever separated or divorced? | |||
| ACE Score | |||
| 0 | 34.8 | 38.3 | 36.4 |
| 1 | 24.7 | 28.0 | 26.2 |
| 2 | 25.8 | 24.6 | 25.2 |
| ≥3 | 14.7 | 9.1 | 12.1 |
ADs
Case findings for AD were based on hospitalizations after the baseline appointment date (prospective phase) with identified International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for 21 ADs in hospital discharge data. Research recommendations and previous studies suggested that AD should be considered as a set of illnesses when taking a public health approach to studying the diseases (1,2,41). For the present study, AD included the following 21 illnesses identified by a recent National Institutes of Health report (1): Addison's disease, autoimmune hemolytic anemia, autoimmune thrombocytopenia purpura, celiac disease, dermatomyositis, Graves' disease, Hashimoto's thyroiditis, idiopathic myocarditis, idiopathic pulmonary fibrosis, insulin-dependent diabetes mellitus, irritable bowel disease, multiple sclerosis, myasthenia gravis, pernicious anemia, psoriasis, rheumatoid arthritis, scleroderma, Sjogren disease, systemic lupus erythematosus, vitiligo, and Wegener's granulomatosis. Persons were considered at risk for hospitalization with ICD-9 coded AD as a discharge diagnosis if they were currently enrolled in the HMO at the time of the hospitalization and the hospitalization with ICD-9 coded AD occurred between the baseline survey date and December 31, 2005.
Because various ADs have different immunopathology, we also examined the relationship between the ACE Score and specific AD groupings (8). One AD group with a cell-mediated, Th1-type immunopathology, which is more prevalent in men, included the following: idiopathic myocarditis, idiopathic pulmonary fibrosis, insulin-dependent diabetes mellitus, irritable bowel disease, and Wegener's granulomatosis (7,8,42–44). Another AD group with an antibody-mediated, Th2-type immunopathology that is more prevalent in women included the following: autoimmune thrombocytopenia purpura, dermatomyositis, Graves' disease, Hashimoto's thyroiditis, myasthenia gravis, rheumatoid arthritis, scleroderma, Sjogren disease, and systemic lupus erythematosus (42–44). A subgroup of the Th2 group included specifically rheumatic diseases (dermatomyositis, rheumatoid arthritis, scleroderma, Sjogren disease, and systemic lupus erythematosus) (43,44). The remaining ADs have a mixed Th1/Th2 pathology without a clear difference in prevalence between men and women (43,44).
Statistical Analysis
Previous reports from the ACE Study have shown that forms of childhood abuse and household dysfunction tend to be interrelated and co-occur (24,34,35). Therefore, to examine ACEs as cumulative stress, we created the ACE Score (range = 0–8), which is an integer count of the total number of categories of ACEs reported by respondents. Four levels of ACE exposures (0, 1, 2, or ≥3) were used for examining hospitalizations with any of 21 ADs. Because of the relatively limited number of ADs when examining specific immunopathology types, we also used a three-level ACE Score (0, 1, or ≥2). Analyses were conducted with the summed score as three dichotomous variables (yes/no) with zero ACE exposures as the referent for examination of any of the 21 ADs and two dichotomous variables with zero ACE as the referent for examination of specific immunopathology types. To test for a trend (graded relationship) between the ACE Score and the risk of AD, the ACE Score was also entered as an ordinal variable with adjustment for the demographic covariates (sex, age, and race). The statistical validity and test-retest reliability of the ACE Score has been published previously; Cohen's κ was found to be 0.64, representing good agreement (45).
Cox proportional hazards regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the likelihood of a first hospitalization for AD during follow-up. Survival time in years was calculated from the date of the baseline survey until the first hospitalization for any AD among AD cases or until December 31, 2005 for participants who remained free of AD; no participants died during follow-up. Independent variables in the models for any AD included age at baseline, race (White, non-White), and the ACE Score, which all satisfied the proportional hazards assumptions. For specific AD immunology types as the outcomes, the proportional hazards assumption was satisfied for age, sex, and ACE Score; however, race failed to satisfy the assumption for Th2 and Th2 rheumatic diseases and was removed from the models. In addition, stratification by two age categories (19 to 64 years and ≥65 years) was conducted because ADs tend to peak during the third and sixth decade of life (3).
RESULTS
Characteristics of the Study Population
The study population included 8293 women (54%) and 7064 men (46%). The mean ± standard deviation age was 56 ± 15 years; 23.9% were aged 19 to 44 years, 43.5% were aged 43 to 64 years, and 32.7% were aged ≥65 years. Seventy-six percent of participants were White, and 11% Hispanic, 4% Black, 7% Asian, <1% Native American, and 2% other. This was a highly educated population such that 40% were college graduates; 36% had some college education; 17% were high school graduates (i.e., 12 years education); and only 7% had not completed high school.
The prevalence of each of the eight individual ACEs was as follows: emotional abuse, 10%; physical abuse, 28%; sexual abuse, 21%; household substance abuse, 27%; mental illness in the home, 19%; witnessed domestic violence, 13%; criminal household member, 5%; and parental separation or divorce, 23% (Table 1). Close to two thirds (64%) of the respondents reported at least one ACE; 37% reported ≥2 ACEs (Table 1).
ADs
Overall 372 (2.4%) of hospitalizations with a discharge diagnosis of AD were identified during follow-up. Among the hospital records with an AD diagnosis, 317 (85%) listed a single AD as a discharge diagnosis whereas 15% listed more than one AD (Table 2). The five most common ADs identified were: insulin-dependent diabetes mellitus (23.1%), rheumatoid arthritis (18.8%), autoimmune thrombocytopenia purpura (16.7%), idiopathic pulmonary fibrosis (9.1%), and systemic lupus erythematosus (8.1%).
TABLE 2.
Autoimmune Disease Grouping by Immunopathology and Number of Cases Identified Through Hospital Discharge Records Between Baseline and December 31, 2005 Among 15,357 Adults by Sex: Adverse Childhood Experiences Study 1995 to 2005
| Autoimmune Disease (ICD-9 Code) | Target Organ, Tissue, or Receptor | Number of Cases |
||
|---|---|---|---|---|
| Women | Men | Total | ||
| Autoimmune diseases associated with cell-mediated, Th1-type immunopathology and/ or increased incidence in males | ||||
| Idiopathic myocarditis (422.91) | Heart | 1 | 0 | 1 |
| Idiopathic pulmonary fibrosis (515.0) | Lung | 11 | 23 | 34 |
| Insulin-dependent diabetes mellitus (250.01, 250.03, 250.13, 250.23, 250.41, 250.51, 250.61, 250.81) | Pancreas | 36 | 50 | 86 |
| Irritable bowel disease (556.0 to 556.9) | Gut | 3 | 9 | 12 |
| Wegner granulomatosis (446.4) | Arteries | 2 | 0 | 2 |
| Autoimmune diseases with mixed Th1/ Th2 responses | ||||
| Pernicious anemia (281.0) | Stomach | 4 | 13 | 17 |
| Vitiligo (709.01) | Skin | 0 | 1 | 1 |
| Addison disease (255.4) | Adrenal glands | 5 | 4 | 9 |
| Autoimmune hemolytic anemia (283.0) | Red blood cells | 3 | 6 | 9 |
| Psoriasis (696.0) | Skin | 7 | 6 | 13 |
| Celiac disease (579.0) | Gut | 3 | 6 | 9 |
| Multiple sclerosis (340.0) | Nerves | 12 | 5 | 17 |
| Autoimmune diseases associated with antibody-mediated, Th2-type immunopathology and/or increased incidence in females (includes rheumatic diseases below) | ||||
| Graves' disease (242.0) | Receptor | 13 | 4 | 17 |
| Autoimmune thrombocytopenia purpura (287.0) | Platelets | 25 | 37 | 62 |
| Myasthenia gravis (358.0) | Receptor | 5 | 5 | 10 |
| Hashimoto's thyroiditis (245.2) | Thyroid | 8 | 0 | 8 |
| Th2-type rheumatic diseases subgroup | ||||
| Scleroderma (710.1) | Collagen | 8 | 0 | 8 |
| Dermatomyositis (710.3) | Muscle | 1 | 1 | 2 |
| Systemic lupus erythematosus (710.0) | Nucleus | 26 | 4 | 30 |
| Sjogren disease (710.2) | Glands | 9 | 0 | 9 |
| Rheumatoid arthritis (714.0) | Joints | 48 | 22 | 70 |
ICD-9 = International Classification of Diseases, Ninth Revision; Th1 = T-helper 1; Th2 = T-helper 2.
Among the 372 hospital records with any diagnosis of an autoimmune disease, 15% include more than one autoimmune disease.
The unadjusted rates (per 10,000 person-years) for AD hospitalizations increased with increasing age (8.8 for ages 19 to 44 years; 26.0 for ages 45 to 64 years, and 56.8 for ages ≥65 years (p < .05)). Because of the expected sex differences in the prevalence of certain ADs (8,42–44), we examined the age-specific relationship between sex and any AD and found that, for persons aged 19 to 64 years, women were 50% more likely than men to be hospitalized with an AD (HR = 1.5; 95% CI = 1.1–2.2); among persons ≥65 years, women were less likely than men to have a first hospitalization with AD (HR = 0.7; 95% CI = 0.5–0.9). There were no race differences in hospitalizations for ADs.
ACE Score and Risk of Hospitalizations for Any AD
We examined the sex-specific relationship between ACEs and the likelihood of hospitalization with any AD (Table 3). For both men and women, the likelihood of a first hospitalization for any AD was higher among adults with 2 or ≥3 ACEs compared with those with no ACE; however, the relationship was statistically significant only for women (p < .05) (Table 3). A test for linear trend was also performed, using the ACE Score as an ordinal variable in the models. For every increase in the ACE Score, the likelihood of a first hospitalization with any AD increased 20% (p < .001) for women and 10% for men (p < .05).
TABLE 3.
Association Between ACE Score and First Hospitalization of Any of 21 Autoimmune Diseases Identified Through Hospital Discharge Records Between Baseline and December 31, 2005 Among 15,357 Adults by Sex: Adverse Childhood Experiences Study 1995 to 2005
| Person-Years | Hospitalizations With Autoimmune Diseases (No.)a | Unadjusted Rate (per 10,000 Person-Years) | Adjusted HR (95% CI)b | ||
|---|---|---|---|---|---|
| Women | 61,688 | 194 | 31.4 | ||
| ACE Score | 0 | 22,010 | 69 | 31.3 | 1.0 (referent) |
| 1 | 15,511 | 37 | 23.8 | 01.4 (1.0–2.1) | |
| 2 | 15,572 | 53 | 34.0 | 2.1 (1.4–3.2) | |
| ≥3 | 8596 | 35 | 40.7 | 2.1 (1.4–3.2) | |
| Men | 51,723 | 178 | 34.4 | ||
| ACE Score | 0 | 20,148 | 69 | 34.2 | 1.0 (referent) |
| 1 | 14,472 | 46 | 31.8 | 1.5 (1.0–2.2) | |
| 2 | 12,642 | 49 | 38.8 | 1.6 (0.9–2.9) | |
| ≥3 | 4460 | 14 | 31.4 | 1.6 (0.9–2.9) |
ACE = adverse childhood experience; HR = hazard ratio; CI = confidence interval.
Autoimmune diseases: Addison's disease, autoimmune hemolytic anemia, autoimmune thrombocytopenia purpura, celiac disease, dermatomyositis, Graves' disease, Hashimoto's thyroiditis, idiopathic myocarditis, idiopathic pulmonary fibrosis, insulin-dependent diabetes mellitus, irritable bowel disease, multiple sclerosis, myasthenia gravis, pernicious anemia, psoriasis, rheumatoid arthritis, scleroderma, Sjogren disease, systemic lupus erythematosus, vitiligo, and Wegener granulomatosis.
HR and 95% CI obtained from sex-specific Cox proportional hazards regression model that included ACE score, age, and race.
When stratified by age, the relationship between the ACE Score and a hospitalization with any of the 21 ADs was stronger among adults aged 19 to 64 years than it was for the cohort aged ≥65 years (Figure 1). A test for linear trend was performed, using the ACE Score as an ordinal variable in the models. The results indicated that, for every increase in the ACE Score, the likelihood of a hospitalization with AD increased 20% for persons aged 19 to 64 years (p < .05) whereas it increased only 10% for ages ≥65 years (p = .08).
Figure 1.
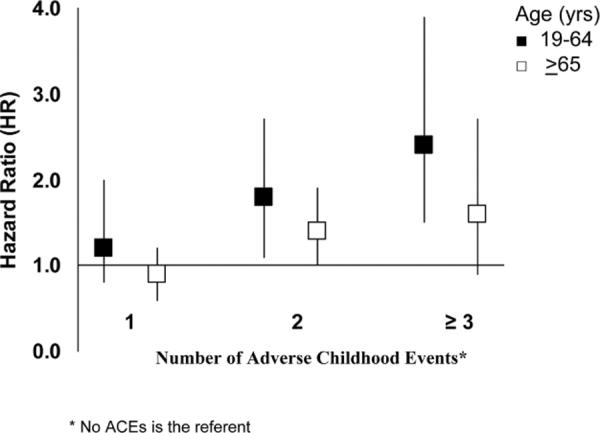
Adjusted hazard ratios and 95% confidence intervals for the association of adverse childhood events with the development of any auto-immune disease, by age groups: Adverse Childhood Experiences Study 1995-2005.
Specific Autoimmune Groupings
Women were 40% less likely than men (p<.05) to have hospitalizations for ADs associated with Th1-type immunopathology (Table 4). However, women had a 50% greater (p < .05) likelihood than men for hospitalization with a Th2-type AD (Table 4) and were also more than twice as likely as men to have a hospitalization for a Th2 specific rheumatic disease (HR = 2.5; 95% CI = 1.6–3.9) (Table 4). Compared with persons with no ACEs, those with ≥2 ACEs were at a 70% increased likelihood for hospitalization with Th1-type ADs (p < .05), 80% increased likelihood for Th2 type ADs (p < .05), and twice the likelihood for hospitalization with Th2 rheumatic diseases (p < .05), after controlling for sex and age (Table 4). The test for linear trend revealed a 20% increased risk for Th1-type AD, 20% increased risk for Th2-type AD, and 30% increased risk for rheumatic diseases for every level of increase in the ACE Score. No significant relationship between ACEs and hospitalizations with mixed Th1/Th2-type AD was observed.
TABLE 4.
Associations of Sex and Baseline ACE Score to First Hospitalization With Selected Autoimmune Disease (Immunopathology) Types Among 15,357 adults: Adverse Childhood Experiences Study 1995 to 2005
| Immunopathology Type | Person-Years | Hospitalizations With Autoimmune Diseases (n) | Unadjusted Rate (per 10,000 Person-Years) | Adjusted HR (95% CI)e,f | ||
|---|---|---|---|---|---|---|
| Th1a | ACE Score | Total | 113,411 | 133 | 11.7 | |
| Men | 51,723 | 82 | 15.8 | 1.0 (referent) | ||
| Women | 61,688 | 51 | 8.3 | 0.6 (0.4–0.8) | ||
| 0 | 42,158 | 49 | 11.6 | 1.0 (referent) | ||
| 1 | 29,983 | 30 | 10.0 | 1.0 (0.6–1.6) | ||
| ≥2 | 41,270 | 54 | 13.1 | 1.7 (1.2–2.5) | ||
| Mixed Th1/Th2c | ACE Score | Total | 113,411 | 74 | 6.5 | |
| Men | 51,723 | 40 | 7.7 | 1.0 (referent) | ||
| Women | 61,688 | 34 | 5.5 | 0.8 (0.5–1.2) | ||
| 0 | 42,158 | 28 | 6.6 | 1.0 (referent) | ||
| 1 | 29,983 | 21 | 7.0 | 1.2 (0.7–2.1) | ||
| ≥2 | 41,270 | 25 | 6.1 | 1.2 (0.7–2.1) | ||
| Th2b | ACE Score | Total | 113,411 | 188 | 16.6 | |
| Men | 51,723 | 69 | 13.3 | 1.0 (referent) | ||
| Women | 61,688 | 119 | 19.3 | 1.5 (1.1–2.0) | ||
| 0 | 42,158 | 67 | 15.9 | 1.0 (referent) | ||
| 1 | 29,983 | 40 | 13.3 | 1.8 (1.3–2.4) | ||
| ≥2 | 41,270 | 81 | 20.0 | 1.8 (1.3–2.4) | ||
| Th2 rheumaticd | ACE Score | Total | 113,411 | 96 | 8.5 | |
| Men | 51,723 | 25 | 4.8 | 1.0 (referent) | ||
| Women | 61,688 | 70 | 11.3 | 2.5 (1.6–3.9) | ||
| 0 | 42,158 | 34 | 8.1 | 1.0 (referent) | ||
| 1 | 29,983 | 14 | 4.7 | 2.0 (1.3–3.2) | ||
| ≥2 | 41,270 | 48 | 11.6 | 2.0 (1.3–3.2) |
ACE = adverse childhood experience; HR = hazard ratio; CI = confidence interval; Th1 = T-helper 1; Th2 = T-helper 2.
Th1 = idiopathic myocarditis, idiopathic pulmonary fibrosis, insulin-dependent diabetes mellitus, irritable bowel disease, and Wegener granulomatosis.
Th2 = autoimmune thrombocytopenia purpura, dermatomyositis, Graves' disease, Hashimoto's thyroiditis, myasthenia gravis, rheumatoid arthritis, scleroderma, Sjogren disease, and systemic lupus erythematosus.
Mixed Th1/Th2 = pernicious anemia, vitiligo, Addison disease, autoimmune hemolytic anemia, psoriasis, celiac disease, multiple sclerosis.
Th2 rheumatic subgroup of Th2 = dermatomyositis, rheumatoid arthritis, scleroderma, Sjogren disease, and systemic lupus erythematosus.
Cox proportional hazard regression model included sex, age, race, and ACE score for analyses of Th1 and mixed Th1/Th2.
Race excluded from the model because it did not meet the proportional hazards assumption for total Th2 and the Th2 rheumatic subgroup.
DISCUSSION
We had the unique opportunity to examine the relationship between ACEs and the likelihood of hospitalizations with AD identified through hospital discharge records. As the number of ACEs increased, the likelihood of hospitalizations with Th1, Th2, Th2-rheumatic, and any of 21 types of ADs also increased. Moreover, the relationship between the ACE Score and AD hospitalizations was stronger among younger adults. To our knowledge, this study is the first to demonstrate a relationship between multiple types of childhood adversity on hospitalizations for AD during adulthood.
Research on nervous, endocrine, and immune interactions has revealed that these systems are anatomically and functionally interconnected (11–13). Stressors, such as infections, toxins, and/or psychological trauma, stimulate the hypothalamic-pituitary-adrenal axis to release corticoid-releasing hormone (CRH), resulting in elevated systemic levels of corticosteroids, such as glucocorticoids. Acute stress initially may increase inflammation through acute-phase mediators like IL-1, IL-6, and CRP that are eventually downregulated by glucocorticoids thereby maintaining homeostasis (46). Chronic stress has the opposite effect and decreases glucocorticoid levels. A recent epidemiologic study confirmed the link between childhood abuse and long-term changes in immune response (36); in this longitudinal study, childhood abuse was associated with elevated CRP levels, white blood cell counts, and other markers of inflammation 20 years later (36).
Women and female rodents have higher systemic baseline levels of glucocorticoids than their male counterparts due to the transcriptional regulation of CRH by estrogen (11). In a similar manner, estrogen increases IL-4 levels in females, resulting in a greater Th2-type immune response (7). Increased glucocorticoid levels in females further enhance IL-4 production (11–13). In contrast, testosterone reduces glucocorticoid and IL-4 levels in males, resulting in a predominantly IFN-γ, Th1-type immune response to infection or trauma (11). Research in rodents (6,42,47,48) and the observed immunopathology and sex prevalence of certain ADs (8,43,44) suggests that sex-specific immune mechanisms may account for the observed differences. In our study, hospitalizations for Th1-associated ADs were more common in men in this cohort, whereas hospitalizations for Th2-associated ADs, such as rheumatoid disorders, were more common in women. This is the first epidemiological study confirming this immunopathologic relationship.
Rheumatic diseases are a group of inflammatory disorders in which autoantibodies and immune complex deposition produce tissue damage (44). One of the characteristics of rheumatic diseases is the production of rheumatoid factor (RF), which is an autoantibody that binds other antibodies. RF is usually produced following viral infections (44), suggesting that infections may contribute to the development of AD (49). Fairweather and Frisancho-Kiss have found that social stress occurring before viral infection in rodents increased inflam matory heart disease in both sexes, but especially in females (unpublished results). These findings and the present study suggest that childhood stressful events may increase ADs independently as well as amplify the effect of other environmental factors, such as infections. Thus, a possible explanation for the increased prevalence of ADs in females is that females respond to similar stressful events differently than males due to sex differences in their physiology and neurobiology (i.e., greater Th2 and glucocorticoid levels that are further amplified by stress) (13,50).
In addition, physiological and anatomical changes in the brains of individuals who have experienced childhood abuse have been documented. For example, Teicher et al. conducted electroencephalograms to measure limbic irritability and found the percentage of clinically significant brain-wave abnormalities to be higher among individuals who had a history of early trauma versus those who did not experience early trauma (51). Magnetic resonance imaging has revealed reductions in hippocampal volumes among severely sexually abused women, and reductions in the intracranial and cerebral volumes among maltreated children compared with non-abused individuals (51–53).
Although the effects cannot be defined to any specific area of the brain, it has been shown that the limbic system, which is responsible for emotional response, is adversely affected. Because ACEs rarely occur in isolation (14,15), the cumulative effect of multiple ACEs shown in our study may have an even more powerful negative effect on a young child's developing brain via repeated activation of the stress response. This repeated “dosing” of the developing central nervous system by adrenal catecholamines and corticosteroids may contribute to central nervous system- and endocrine-mediated differences in immune function that result in an increased risk for AD.
There are several limitations to the present study. Inflammatory biomarkers, such as CRP or white blood cell counts, were not compared with ACE Scores because biological samples were not available. Our data cannot provide certainty about the temporal relationship between stress exposure (ACEs) and AD, because of the lack of information about the age at which ACEs occurred and also lack of information on the age at onset of the AD. However, given the age at onset for most ADs, it is likely that the ACEs antedated disease onset in most cases. Also, the lack of information about the exact age at which ACEs occurred potentially limits any specific inferences that might be made about the developmental pathway of ACEs on AD.
There are potential limitations with retrospective reporting of childhood experiences. Respondents may have difficulty recalling certain events. However, longitudinal follow-up of adults whose childhood abuse was documented has shown that their retrospective reports of childhood abuse are likely to underestimate actual occurrence (54,55). Difficulty recalling childhood events likely results in misclassification (classifying persons truly exposed to ACEs as unexposed) that would bias our results toward the null (56). This potential weakness may result in underestimates of the true strength of the relationships between ACEs and hospitalization for AD.
ADs were identified through hospitalizations and not outpatient data. Future studies may be strengthened through the use of clinical data because most ADs are diagnosed through outpatient visits. Finally, it is possible that other unmeasured factors at the time of hospitalizations that were not included in our analyses could have affected the strength of our estimates (either upward or downward) of association between ACEs and ADs. Despite this weakness, our data provide preliminary evidence of the association between ACEs and ADs.
It is important to note that the prevalence estimates for childhood exposures we report are nearly identical to those reported in surveys of the general population. We found that 16% of the men and 25% of the women met the case definition for contact sexual abuse; a national telephone survey of adults in 1990 conducted by Finkelhor et al., using similar criteria, estimated that 16% of men and 27% of women had been sexually abused (57). As for physical abuse, 28% of the men from our study had experienced this abuse as boys, which closely parallels the percentage (31%) found in a population-based study of Ontario men that used questions from the same scales (58). The similarity of the estimates from the ACE study to those of population-based studies suggests that our findings are likely to be applicable in other settings.
This is the first study to find an association between early childhood stressors and the development of AD decades later. Our epidemiological findings, coupled with the documented immunopathology of AD, provide preliminary evidence of the relationship between early childhood stress with the human physiological and immunological response, which may also contribute to and expand on the theory of developmental origins of adult disease and health (59). Because childhood adverse events are common and ADs are chronic and often debilitating, expansion of research in this area may further elucidate the impact of stress on adult chronic disorders such as ADs.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The Adverse Childhood Experiences Study was supported under cooperative agreement #TS-44 to 10/11 from the Centers for Disease Control and Prevention through the Association of Prevention Teaching and Research and a grant from the Garfield Memorial Fund. This study was also partially supported through a sole source contract (#200-2005-M-13275) with the Kaiser Foundation Research Institute. Dr. Fairweather is supported by Grant R01 HL087033 from the National Heart, Lung and Blood Institute.
Glossary
- ACE
adverse childhood experience
- AD
autoimmune disease
- Th1
T-helper 1
- Th2
T-helper 2
- CRP
C-reactive protein
- CRH
corticoid releasing hormone
REFERENCES
- 1.United States Department of Health and Human Services Progress in Autoimmune Diseases Research, Report to Congress, National Institutes of Health, The Autoimmune Diseases Coordinating Committee; Mar, 2005. Publication No. 05-5140. [Google Scholar]
- 2.Jacobsen DL, Gange SJ, Rose NR, Graham NMH. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3.Rosen A, Warrell DA, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. 4th ed Oxford University Press; New York, NY: 2003. Autoimmunity; pp. 151–159. [Google Scholar]
- 4.Dooley MA, Hogan SL. Environmental epidemiology and risk factors for autoimmune disease. Curr Opin Rheumatol. 2003;15:99–103. doi: 10.1097/00002281-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Fairweather D. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd; Chichester, UK: Jan, 2007. Autoimmune disease: mechanisms. http://www.els.net/ [DOI: 10.1002/9780470015902.a0020193] [Google Scholar]
- 6.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Rose NR, Fairweather D. Cutting Edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 7.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 8.Fairweather D, Frishancho-Kiss S, Rose NR. Sex differences in autoimmune diseases from a pathological perspective. Americal Journal of Pathology. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113:224–230. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 11.Cutolo M, Capellina S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. Estrogens and autoimmune diseases. Ann NY Acad Sci. 2006;1089:538–547. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 12.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines and autoimmunity. Ann NY Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 13.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and –adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 14.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. The relationship of adult health status to childhood abuse and household dysfunction. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 15.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. J Am Med Assoc. 1999;82:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 16.Dietz PM, Spitz AM, Anda RF, Williamson DF, McMahon PM, Santelli JS, Nordenberg DF, Felitti VJ, Kendrick JS. Unintended pregnancy among adult women exposed to abuse or household dysfunction during their childhood. J Am Med Assoc. 1999;82:1359–1364. doi: 10.1001/jama.282.14.1359. [DOI] [PubMed] [Google Scholar]
- 17.Hillis SD, Anda RF, Felitti VJ, Nordenberg D, Marchbanks PA. Adverse childhood experiences and sexually transmitted diseases in men and women: a retrospective study. Pediatrics. 2000;106:E11. doi: 10.1542/peds.106.1.e11. [DOI] [PubMed] [Google Scholar]
- 18.Hillis SD, Anda RF, Felitti VJ, Marchbanks PA. Adverse childhood experiences and sexual risk behaviors in women: a retrospective cohort study. Fam Plann Perspect. 2001;33:206–211. [PubMed] [Google Scholar]
- 19.Anda RF, Felitti VJ, Chapman DP, Croft JB, Williamson DF, Santelli J, Dietz PM, Marks JS. Abused boys, battered mothers, and male involvement in teen pregnancy. Pediatrics. 2001;107:e19. doi: 10.1542/peds.107.2.e19. [DOI] [PubMed] [Google Scholar]
- 20.Dube SR, Anda RF, Felitti VJ, Chapman D, Williamson DF, Giles WH. Childhood abuse, household dysfunction and the risk of attempted suicide throughout the life span: findings from Adverse Childhood Experiences Study. J Am Med Assoc. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- 21.Dube SR, Anda RF, Felitti VJ, Edwards VJ, Croft JB. Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav. 2002;27:713–725. doi: 10.1016/s0306-4603(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 22.Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti VJ. Body weight, obesity, and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- 23.Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, Williamson DF. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv. 2002;53:1001–1009. doi: 10.1176/appi.ps.53.8.1001. [DOI] [PubMed] [Google Scholar]
- 24.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the Adverse Childhood Experience Study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 25.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 26.Dong M, Anda RF, Dube SR, Felitti VJ, Giles WH. Adverse childhood experiences and self-reported liver disease: new insights into a causal pathway. Arch Intern Med. 2003;163:1949–1956. doi: 10.1001/archinte.163.16.1949. [DOI] [PubMed] [Google Scholar]
- 27.Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, Marks JS. The association between adolescent pregnancy, long-term psychosocial outcomes, and fetal death. Pediatrics. 2004;113:320–327. doi: 10.1542/peds.113.2.320. [DOI] [PubMed] [Google Scholar]
- 28.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: the Adverse Childhood Experiences Study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 29.Chapman DP, Anda RF, Felitti VJ, Dube SR, Edwards VJ, Whitfield CL. Epidemiology of adverse childhood experiences and depressive disorders in a large health maintenance organization population. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Dube SR, Anda RF, Whitfield CL, Brown DW, Felitti VJ, Dong M, Giles WH. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. 2005;28:430–438. doi: 10.1016/j.amepre.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF. Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. J Adolesc Health. 2006;38:444.e1–444.e10. doi: 10.1016/j.jadohealth.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Anda RF, Felitti VJ, Walker J, Whitfield CL, Bremner JD, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psych Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards VJ, Anda RF, Gu D, Dube SR, Felitti VJ. Adverse childhood experiences and smoking persistence in adults with smoking-related symptoms and illness. Permanente J. 2007;11:5–7. doi: 10.7812/tpp/06-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dube SR, Anda RF, Felitti VJ, Croft JB, Edwards VJ, Giles WH. Growing up with parental alcohol abuse: exposure to childhood abuse, neglect, and household dysfunction. Child Abuse Neglect. 2001;25:1627–1640. doi: 10.1016/s0145-2134(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 35.Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Neglect. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Nat Acad Sci. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szalai AJ. C-reactive protein (CRP) and autoimmune disease: facts and conjectures. Clin Dev Immunol. 2004;11:221–226. doi: 10.1080/17402520400001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straus M, Gelles RJ. Physical violence in American families: risk factors and adaptations to violence in 8,145 families. Transaction Press; New Brunswick, NJ: 1990. [Google Scholar]
- 39.Wyatt GA. The sexual abuse of Afro-American and white American women in childhood. Child Abuse Negl. 1985;9:507–19. doi: 10.1016/0145-2134(85)90060-2. [DOI] [PubMed] [Google Scholar]
- 40.Schoenborn CA. Exposure to alcoholism in the family: United States, 1988. Adv Data. 1991;205:1–13. [PubMed] [Google Scholar]
- 41.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiological studies. Ann NY Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 42.Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10:2005–2011. doi: 10.3201/eid1011.040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose NR, Mackay IR, editors. The Autoimmune Diseases. 4th ed. Elsevier Academic Press; St. Louis, MO: 2006. [Google Scholar]
- 44.Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Elsevier Saunders; Philadelphia, PA: 2005. [Google Scholar]
- 45.Dube SR, Williamson DF, Thompson T, Felitti VJ, Anda RF. Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse Neglect. 2004;28:729–737. doi: 10.1016/j.chiabu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 46.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 47.Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, Fairweather D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rβ1 signaling and IFN-γ increase inflammation in males independent from STAT4. Brain Res. 2006;1126:139–147. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Gatewood SJL, Davis SE, Njoku DB, Rose NR. IFN-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from coxsackievirus-induced myocarditis. Rev Med Virol. 2005;15:17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- 50.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psych Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 51.Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann NY Acad Sci. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 52.Perry BD, Pollard RA, Blakely TL, Baker WL, Vigilante D. Childhood trauma, the neurobiology of adaptation and use-dependent development of the brain: how states become traits. Infant Mental Health J. 1995;16:281–291. [Google Scholar]
- 53.Van der Kolk BA, Fisler RE. Childhood abuse and neglect and loss of self-regulation. Bull Menninger Clin. 1994;58:145–168. [PubMed] [Google Scholar]
- 54.Femina DD, Yeager CA, Lewis DO. Child abuse: adolescent records vs. adult recall. Child Abuse Negl. 1990;14:227–231. doi: 10.1016/0145-2134(90)90033-p. [DOI] [PubMed] [Google Scholar]
- 55.Williams LM. Recovered memories of abuse in women with documented child sexual victimization histories. J Trauma Stress. 1995;8:649–673. doi: 10.1007/BF02102893. [DOI] [PubMed] [Google Scholar]
- 56.Rothman KJ. Modern epidemiology. Little, Brown; Boston: 1986. [Google Scholar]
- 57.Finkelhor D, Hotaling G, Lewis IA, Smith C. Sexual abuse in a national survey of adult men and women: prevalence, characteristics, and risk factors. Child Abuse Negl. 1990;14:19–28. doi: 10.1016/0145-2134(90)90077-7. [DOI] [PubMed] [Google Scholar]
- 58.MacMillan HL, Fleming JE, Trocme N, Boyle MH, et al. Prevalence of child physical and sexual abuse in the community: results from the Ontario Health Supplement. JAMA. 1997;278:131–5. [PubMed] [Google Scholar]
- 59.Lawlor DA. The developmental origins of health and disease: Where do we go from here? Epidemiology. 2008;19(2):206–208. doi: 10.1097/EDE.0b013e3181635ddc. [DOI] [PubMed] [Google Scholar]


