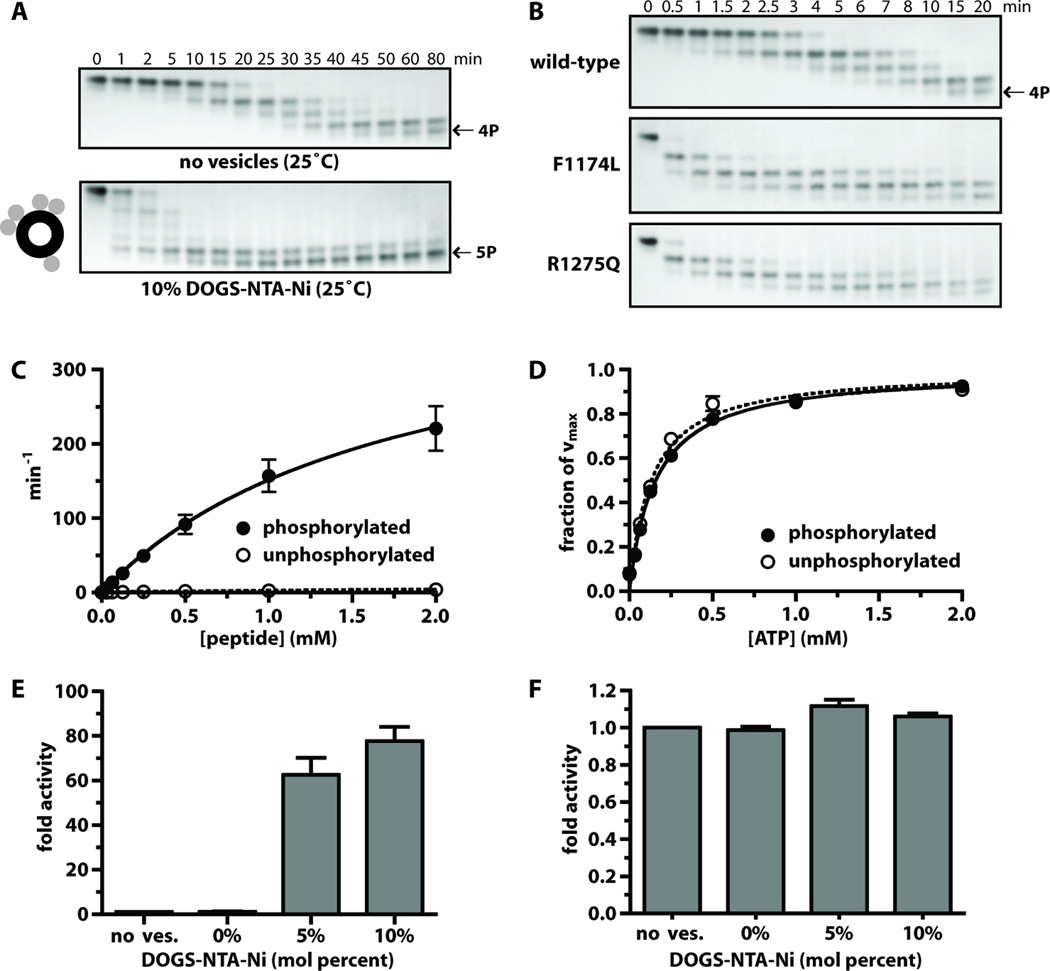
Fig. 4. Analysis of ALK-TKD activation in vitro.
(A) Separation of differently autophosphorylated ALK-TKD species by native gel electrophoresis to monitor autophosphorylation at 25°C in the absence (top) and presence (bottom) of vesicles containing 10% DOGS-NTA-Ni (100µM total lipid), 10mM MgCl2, and 2mM ATP. (B) Autophosphorylation of wild-type and mutated ALK-TKD (10µM) with saturating ATP (2mM) and 10mM MgCl2 at 37°C. Results are quantitated in fig. S4. (C) Rate of 32P incorporation at 25°C into substrate peptide (see Materials and Methods) for autophosphorylated ALK-TKD (10nM) and unphosphorylated ALK-TKD (500nM) as peptide substrate concentration is increased. ATP concentration was 2mM. (D) Km, ATP determination for autophosphorylated (10nM) and unphosphorylated (500nM) ALK-TKD at fixed peptide substrate concentration (1mM). (E) Enhancement of EGFR-TKD kinase (1µM) by vesicles containing increasing mole percentages of DOGS-NTA-Ni (10µM DOGS-NTA-Ni, 100–200µM total lipid). (F) Effect on autophosphorylated ALK-TKD (1µM) activity of adding DOGS-NTA-Ni vesicles. Data are shown as means ± SEM from at least three independent experiments. All experiments except those in (B) were performed at 25°C.