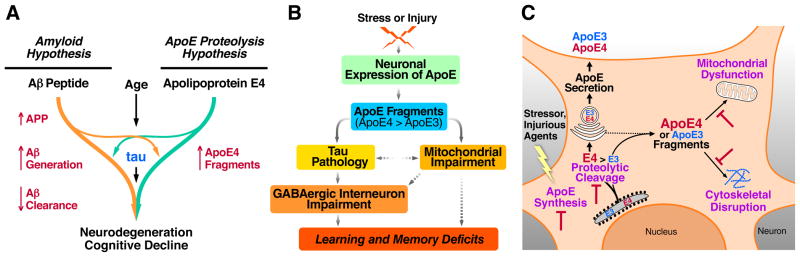
Figure 3. ApoE4 in AD Pathogenesis and Related Therapeutic Strategies.
(A) ApoE4 probably has both Aβ-dependent and Aβ-independent roles in AD pathogenesis. ApoE4 impairs Aβ clearance and promotes Aβ deposition (left). In addition, neuronal apoE4 undergoes proteolytic cleavage to generate neurotoxic fragments, contributing to AD pathogenesis independently of Aβ stresses or injuries, neuronal apoE expression is triggered to facilitate neuronal repair. However, neuronal apoE undergoes proteolytic cleavage, with apoE4 being more susceptible to the cleavage than apoE3, resulting in the formation of neurotoxic fragments. The apoE4 fragments enter the cytosol and cause tau pathology and mitochondrial impairment. Hilar GABAergic interneurons in the dentate gyrus are particularly vulnerable to apoE4 fragment toxicity and the resulting impairments contribute to learning and memory deficits. (C) Neuronal expression of apoE and apoE proteolysis-related neurotoxicity represent targets for the development of novel therapeutics. Inhibiting apoE4 expression in neurons should reduce the level of toxic apoE4 fragments and their downstream detrimental effects. Identification of the protease that cleaves apoE in neurons should enable the development of specific protease inhibitors to reduce the production of neurotoxic apoE fragments. It may also be possible to develop drugs to protect mitochondria and the cytoskeleton from attack by the toxic apoE fragments.