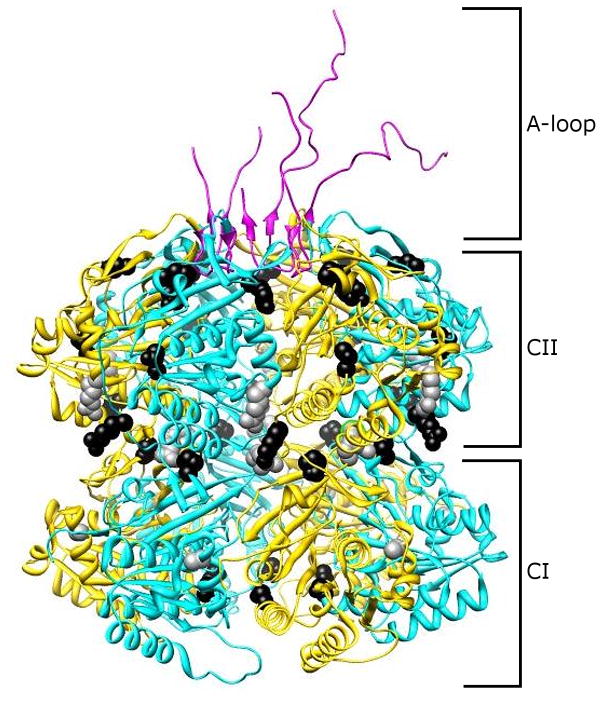
Figure 1.
Period-altering amino acid substitutions mapped to structure of a KaiC hexamer. KaiC monomers consist of two tandemly duplicated domains, CI and CII. These monomers oligomerize into hexameric structures with a thin “waist” region connecting the CI and CII regions. At the C-terminus of each KaiC monomer exists an “A-loop,” which determines the steady-state level of KaiC phosphorylation. Amino acid substitutions in the KaiC protein (Ishiura et al., 1998) that result in short-period rhythms (A87V, R215C, and R321Q) are denoted by gray circles, while those substitutions that give rise to period lengths greater than that of wild-type cells (S157C, P236S, R253H, M273I, T409A, and Y442H) are depicted as black circles. Image provided by Yong-Ick Kim, UC-San Diego.