Abstract
Social environment can affect the expression of sex-typical behavior in both males and females. Males of the African cichlid species Astatotilapia burtoni have long served as a model system to study the neural, endocrine, and molecular basis of socially plastic dominance behavior. Here we show that in all-female communities of A. burtoni, some individuals acquire a male-typical dominance phenotype, including aggressive territorial defense, distinctive color patterns, and courtship behavior. Furthermore, dominant females have higher levels of circulating androgens than either subordinate females or females in mixed-sex communities. These male-typical traits do not involve sex change, nor do the social phenotypes in all-female communities differ in relative ovarian size, suggesting that factors other than gonadal physiology underlie much of the observed variation. In contrast to the well-studied situation in males, dominant and subordinate females do not differ in the rate of somatic growth. Dominant females are not any more likely than subordinates to spawn with an introduced male, although they do so sooner. These results extend the well known extraordinary behavioral plasticity of A. burtoni to the females of this species and provide a foundation for uncovering the neural and molecular basis of social dominance behavior while controlling for factors such as sex, gonadal state and growth.
Keywords: Aggression, social dominance, sex steroid hormones, reproduction, growth
Introduction
Aggressiveness varies among species, populations, environments, life stages, sexes, and individuals. Understanding the proximate causes of variation in aggressiveness is necessary in order to understand the evolution of social behavior and to explain mechanisms causing the observed trade-offs between aggressiveness and other fitness-linked traits (Knapp et al., 1999; McGlothlin and Ketterson, 2008). However, important physiological or experimental limitations often confound mechanistic studies of aggressiveness. It is often impossible to know to what extent molecular and mechanistic differences between individuals displaying varying levels of aggressiveness are directly related to the observed behavioral phenotype. While not independent of the behavior, these associated physiological changes complicate efforts to identify the mechanisms underlying variation in aggressiveness. Studying aggressiveness in alternate contexts might allow us to control some of these potentially confounding factors.
Social fish species have emerged as important model systems for the study of aggressive behavior and its underlying physiological mechanisms (Arnott and Elwood, 2009). The African cichlid fish Astatotilapia burtoni has become a particularly powerful model system in social neuroscience (for review: (Fernald, 2004; Robinson et al., 2008; Wong and Hofmann, 2010), while at the same time its ecology and behavior have been well characterised in nature (Fernald and Hirata, 1977a, b). We aim to use social hierarchies within experimentally manipulated female communities to experimentally dissociate the physiological and molecular mechanisms underlying social dominance from some of the potentially confounding variables, such as sex, gonadal state, and growth rate.
At any given time, males of A. burtoni assume one of two social states (Hofmann, 2003). Dominant males are colourful and display a characteristic melanocyte-based lachrymal stripe across their face (so-called eye-bar). They aggressively maintain territories from which they court females. In addition to actual physical encounters, dominant males exhibit ritualized displays of aggression directed at subordinate males and other dominant males. The dominant males have elevated levels of testosterone and 11-KT, and they are reproductively active, producing sperm and spawning with females to fertilize eggs. Subordinate males do not hold a territory, look and act like females, have low androgen levels, and are reproductively inactive with regressed gonads. Individuals shift between dominant and subordinate states throughout life, in part due to differential growth rates (Hofmann, 2000; Hofmann et al., 1999; Parikh et al., 2006). Importantly, these transitions can be experimentally induced in order to gain insights into the hormonal and molecular mechanisms underlying these endpoints (Renn et al., 2008) and the transition process (Burmeister, 2005; Maruska and Fernald, 2010).
Female A. burtoni behave much like subordinate males under normal conditions in the field and laboratory, i.e., they school and feed with other females, juveniles, and subordinate males (Fernald, 1977a; Fernald and Hirata, 1977a, b) (White and Fernald, 1993). Once gravid (full with mature eggs), a female will spawn with dominant males, after which she incubates the fertilized eggs in her buccal cavity for several weeks. Upon release of the fry a female may defend a territory and continue to exhibit maternal care for a short period (Renn et al., 2009; Fernald and Hirata, 1977a). Aggressiveness in females provides an alternate paradigm in which to address the mechanistic basis of such behavior.
In the present study, we show that when all males are removed from the social group, some females acquire morphological and behavioral traits typical of the dominant territorial male phenotype, exhibiting a change in their sex-role without undergoing sex change. These aggressive females acquire dark eye-bars, defend territories, with vigorous chasing, threat displays and border threats, and even court and spawn with other females. At the physiological level, these behavioral changes are accompanied by the increase in circulating levels of androgens. Our results establish a powerful experimental system for uncovering the molecular basis of specific behavioral patterns. While not thought to mimic ecologically relevant conditions, the experimental manipulations, behavioral observations and physiological measures presented here establish a foundation for the analysis of female behavior in this important model of social behavior. Furthermore, the ability to study dominance behavior in this alternate context (i.e. in females) presents the opportunity to remove, or reduce the influence of, confounding factors (e.g. gonadal state, somatic growth) that have complicated efforts to specifically relate gene expression to behavior in past mechanistic studies of male behavior (e.g. Renn et al., 2008).
Methods
Housing of Fish
Fish derived from a laboratory stock were kept in 110 L aquaria at 28°C and pH 8.5 under full spectrum 12hr light/12 hr dark with 10 minute dim light periods to simulate dawn and dusk, mimicking the conditions found in the natural habitat in the East African Lake Tanganyika. Fish were fed daily and provided with gravel and terracotta flowerpots to simulate natural shelters positioned in each corner and one near the center. All experiments were conducted in accordance with the animal care and use guidelines of Harvard University (IACUC protocol number 22–22).
Behavioral measures for female A. burtoni
In order to quantify female behavior, we adapted the systematic description previously developed by Fernald (1977a) for dominant and subordinate A. burtoni males. Aggressive behavior patterns included chasing or biting, threatening, and engaging in border threats. Submissive behavior was measured by counting fleeing events triggered by an attacker. Mating behaviors consisted of digging, courting, and spawning. Courting was scored only for male-typical courtship behavior that involves lateral display, tail quiver, and leading another female toward the spawning territory. These behavioral measures were used to calculate a dominance index as the sum of all aggressive behaviors (biting, threatening and border threats) minus the number of fleeing events performed by an individual (Renn et al., 2008; White et al., 2002).
Although spawning was never directly observed during any of the focal observation periods, fish did spawn between observations and these events were recorded based on the visual observation of eggs in the buccal cavity. In addition to behavioral observations we recorded body coloration as either blue, yellow, or grey according to subjective report, and we recorded reproductive status as brooding, non-gravid or gravid by visual inspection of buccal or body distension. Schooling time and time in territory were estimated as the proportion of time the focal female was within 2 body lengths of either the majority of the fish in the tank or the terracotta flowerpot that she normally defended. The proportion of time the characteristic eye-bar was displayed was also estimated as a proportion of the observation period.
Observation Protocol
Three all-female experimental communities were established in 110-liter aquaria, with 9–10 females and 4–5 available pot shard territories each. While mean mass and length varied between communities (mean mass: 4.90g±1.6 s.d. − 7.48g±0.58 s.d.; mean standard length 5.59cm±0.41 s.d. − 6.36cm±0.18 s.d.), the females within a community were roughly size matched. These aquaria were maintained for >90 days and are referred to as the “long-term” observations. Five additional all-female experimental communities were maintained for behavioral measures and neural tissue samples (for gene expressions studies reported elsewhere). Here, each 110-liter aquarium was divided in two by a clear divider and each side contained 5 females and 3 available potshard territories. Here, females were roughly size matched across all five communities (mean of mass: 3.54g±0.66 s.d.; mean standard length: 5.03cm±0.29 s.d.). These communities are referred to as “short-term” as they were maintained for only 45 days. Three mixed-sex control communities comprised of 5–6 males and 5–6 females with 4 pot shard territories were maintained in 110-liter aquaria. Here, the females were roughly size matched across all three communities (mean female mass: 4.19g±0.70 s.d.; mean standard length: 5.12cm±0.13 s.d.). Males were age matched to females, and therefore were larger (not measured). The females in these mixed-sex communities are referred to as control females. Two of these mixed sex communities were observed in parallel with the short-term observations and one in parallel with the long-term observation experiment. In all cases, females of unspecified reproductive cycle were taken from standard stock tanks and individually marked with a colored bead held through the dorsal muscle by a plastic tag (Avery Dennison, Pasadena CA).
In all communities, three-minute real-time focal observations were performed 2–3 times per week between 08:30 and 10:00 hours (0.5–2 hours after light onset) following a 5–10 min. accommodation period during which the observer sat in front of each aquarium. All females in a single tank were observed sequentially on the same day, and the order was varied each observation day. All female fish were surveyed daily for brooding status. Behavioral data from females in both the long-term and short-term observation communities are reported only for those fish that exhibited a consistent behavioral phenotype for the four weeks preceding euthanasia (DOM: n=15; SUB: n=21, Control: n=14) and included four to ten observations per fish (mean = 7.94 observations).
Male Reintroduction
In order to determine whether a female’s mating success was related to her social status we reintroduced males into all-female communities. Three additional 110-liter aquaria were established, each of which housed two communities of 5 females separated by a clear divider. Focal observations were performed twice every week between 08:00 and 10:00 hours for seven weeks in order to identify dominant and subordinate individuals and quantify the total number of days each individual displayed a dominant phenotype. These animals are not included in the detailed behavioral analysis. After seven weeks a single male was introduced to each community. Three times per week, for the following 30 days, females were visually inspected for evidence of eggs in the buccal cavity.
Growth and Condition
At intervals of two to five weeks, fish were weighed and standard length (SL) was recorded. As was already observed by Hofmann et al. (1999), removal of fish for weighing and measuring does not disrupt hierarchies; the behavior patterns displayed before and after each measurement do not differ qualitatively, and dominant females always returned to their original territories. Growth rate (GR) was calculated as the average weekly relative change in SL during the final four weeks of the experiment when the animals’ social status was stable. Condition factor (CF) was calculated based on the residuals from the regression of body mass on standard length at the end of the observation periods for those animals that held a stable social status for the preceding four weeks (i.e. the individuals for which behavior data are also reported). Gonadosomatic index (GSI) was calculated as (gonad mass/body mass) × 100 for the subset of females that had displayed a consistent social phenotype for four weeks and were killed at the end of the short-term observation experiment.
Hormone Assays
Using heparinized butterfly infusion sets (SURFLO 26G, #SV-25BLK), we collected blood from the dorsal aorta from dominant (n=8) and subordinate (n=15) females in the short-term communities as well as from control females (n=6). As for the reported behavioral measures, only individuals that had demonstrated consistent social phenotype for 28 days or longer were used. The plasma was separated from the serum using a tabletop centrifuge at 5000rpm for 10min and stored at −80° C. We measured testosterone with a direct radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX) in plasma samples diluted 1:12.5 (Trainor and Hofmann, 2006). The crossreactivity of the assay for dihydrotestosterone was 5.8% and 2.3% for androstenedione. We measured estradiol in plasma samples diluted 1:10 also using a direct radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX). The sensitivity of both assay systems was 0.1 ng/mL. There was not sufficient sample volume to also measure 11-ketotestosterone. Furthermore, in A. burtoni circulating levels of this fish-specific androgen have always been tightly correlated with testosterone, independent of sex, albeit at much lower concentrations (Kidd et al., 2010; Parikh et al., 2006). For each assay system, we followed the manufacturer’s protocol. For each hormone assay, assayed concentrations for serial dilutions of an A. burtoni plasma pool were compared with standards and computed regression lines did not differ in slope (p > 0.05). All samples were assayed in duplicate in each assay. The intra-assay coefficient of variation was 3.3% for testosterone and 8.4% for estradiol.
Statistical Analysis
All statistical analyses were performed in R, the open source software environment for statistical computing and graphics (R development group). Due to non-normal distribution of the behavioral, hormonal and GSI data, p-values were calculated using one-way ANOVA in a randomization test based on 10,000 replications. To identify significant differences in each pair wise comparison of dominant, subordinate and control females, the probability of obtaining a difference at least as great as observed was calculated from the randomization and those p-values were adjusted for multiple comparisons (Benjamini and Hochberg, 1995). Behavioral data, averaged over the final four weeks, were analyzed together for females in the long-term (DOM: n=9; SUB: n=15, Control: n=12) and short-term (DOM: n=6; SUB: n=6, Control: n=4) observation communities because there were no statistically significant differences between the tanks. Linear Least Squares Regression was applied between hormone measures and Spearman rank correlations were computed to assess the relationship between hormone titers and specific behaviors (averaged over just one week prior to blood collection).
Results
Female Appearance and Behavior Can Resemble that of Dominant Males
In both long-term and short-term all-female communities, a subset of the females displayed an eye-bar approximately 90% of the time, while this characteristic display of social dominance was observed only 10% of the time in the remainder of the females in the all female communities and only 5% of the time in the control females. These females displaying the eye-bar, like dominant males, exhibited either yellow or blue coloration; however, it was not as vivid as is seen in males (Figure 1). The females that displayed the eye-bar 10% of the time or less did not exhibit any body color. The red humeral patch and the distinctive egg spots on the anal fin, which are two other color patterns typical of dominant males, were never seen in any of the females.
Figure 1.

Representative photographs of females expressing either the dominant (left) or subordinate (right) phenotype typical of male social organization. Note the male-typical eye-bar displayed by the dominant female (arrow).
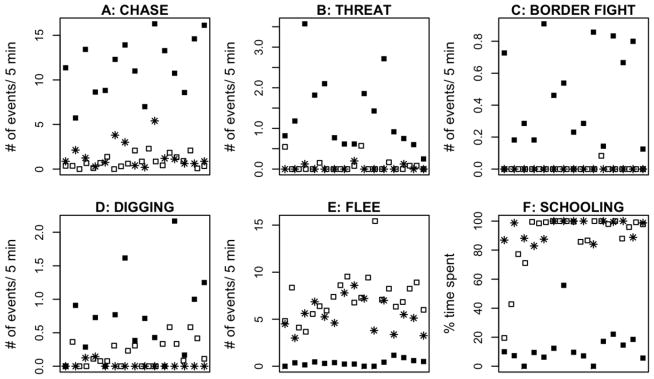
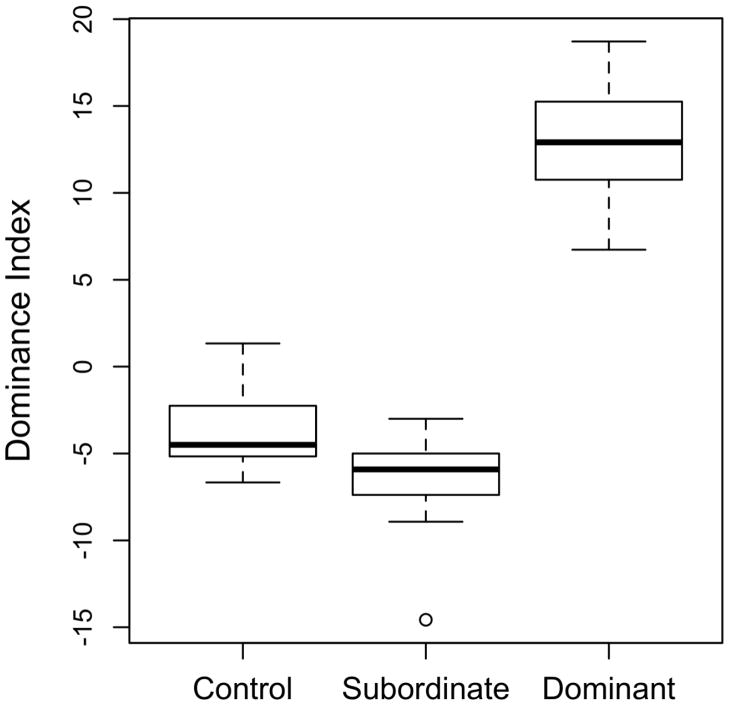
Females that developed these male-typical morphological characteristics also displayed male-typical dominance behavior. Behavioral measures, dominance index and time in territory see below), not color, were used to designate “dominant” females at the end of each observation period. Here we analyze and present the behavior of only those individuals that consistently expressed the same social phenotype for the last four weeks of continuous observation (DOM: n=15; SUB: n=21; Controls: n=14). We found significant differences according to phenotype for several male-typical aggressive displays (Figure 2; Table 1). We observed significantly more chasing events in dominant females compared to either subordinate (p<0.0001) or control females (p<0.0001) (mean number per 3 minute observation ± SE: DOM: 11.45±0.86 SUB: 0.83±0.16 CON: 1.50±0.39). Dominant females also displayed significantly more threat displays compared to either subordinate (p<0.0001) or control females (p<0.0001) (DOM: 1.33±0.25 SUB: 0.08±0.04 Control: 0.03±0.02), and stereotypical border threats were observed almost exclusively in dominant females (DOM: 0.48±0.08 SUB: 0.004±0.005 Control: 0.0±0.0). Dominant females showed a tendency to escalate aggressive interactions to a “carousel” display, a male-typical behavior; however, these events were very rare (data not shown). Furthermore, dominant females were more likely than either subordinate (p=0.0006) or control females (p<0.0001) to perform digging behavior (DOM: 0.69±0.17 SUB: 0.19±0.04 Control: 0.02±0.01), which dominant males display to prepare the territorial bower for spawning. Nine of the 15 dominant females were also observed at least once performing male-typical courtship displays, whereas subordinate females never initiated courtship and only once was a similar behavior observed from a control female in the mixed-sex communities. Much of the aggressive behavior observed in dominant females was directed toward the subordinate females. Dominant females fled significantly less often than either subordinate (p < 0.0001) or control females (p=0.00015) (DOM: 0.38±0.09; SUB: 7.37±0.55; Control: 5.43±0.47) and spent a significantly smaller fraction of their time schooling (DOM: 13.06±3.57%; SUB: 88.49±4.71%; Control: 93.93±1.85). Subordinate females closely resembled control females that were housed with males such that no statistically significant behavioral differences were observed between subordinate and control fish, even the difference in the number of observed fleeing events was not significant (p=0.097). For males, aggressive and subordinate behaviors have been used to calculate a dominance index (Renn et al., 2008; White et al., 2002); see methods). This measure also effectively differentiates the female phenotypes (DOM: 12.75±0.83; SUB: −6.5±0.54; Control: −3.72±0.83; F2,48; p<0.0001; Figure 3). The index for the dominant females is significantly greater than for subordinate or control females (p<0.0001), but was not statistically different for subordinate compared with control females (p=0.3606).
Figure 2.
Behavioral female phenotypes. Dominant (solid squares) females exhibit several male-typical aggressive behavior patterns: (A) Chasing (B) Threat Display (C) Border Threats and (D) Digging. Subordinate (open squares), like control (asterisk), females show a high level of (E) Flee and (F) Schooling. Individual fish are arranged along the x-axes in a consistent but not systematic order.
Table 1.
Behavioral measures averaged over the last four weeks for female phenotypes in both long term and short term observation tanks. (C = Control; D = Dominant; S = Subordinate)
| Behavior | Mean (+s.e.m) | One-way ANOVA | P-value (Tukey HSD) | |||||
|---|---|---|---|---|---|---|---|---|
| Control n=15 | Subor-dinate n=21 | Dominant n=15 | F2,48 | p | C vs. S | C vs. D | S vs. D | |
| Chase | 1.50 (0.396) | 0.837 (0.162) | 11.456 (0.862) | 145.99 | < 0.0001 | 0.7027 | < 0.0001 | < 0.0001 |
|
| ||||||||
| Border Fight | 0 (0) | 0.004 (0.004) | 0.482 (0.079) | 48.09 | < 0.0001 | 0.965 | < 0.0001 | < 0.0001 |
|
| ||||||||
| Threat | 0.03 (0.017) | 0.08 (0.374) | 1.334 (0.246) | 33.06 | < 0.0001 | 0.0501 | < 0.0001 | < 0.0001 |
|
| ||||||||
| Flee | 5.43 (0.466) | 7.378 (0.551) | 0.381 (0.088) | 63.63 | < 0.0001 | 0.0965 | 0.0002 | < 0.0001 |
|
| ||||||||
| Digging | 0.018 (0.013) | 0.187 (0.044) | 0.694 (0.168) | 14.38 | < 0.0001 | 0.2854 | < 0.0001 | 0.0006 |
|
| ||||||||
| School % time | 94.33 (1.846) | 88.493 (4.713) | 13.058 (3.571) | 128.43 | < 0.0001 | 0.6649 | < 0.0001 | < 0.0001 |
|
|
||||||||
Figure 3.
Dominance Index for each female phenotype. Boxplots show the upper and lower quartiles around the median; whiskers indicate the lowest and highest datum still within the 1.5 interquartile range. All pairwise comparisons are significantly different at alpha = 0.05 (see Table 1).
Dominant females displayed many of the morphological and behavioral characteristics normally associated with dominant males, yet, even in the long-term (up to 140 days) all-female communities, no female changed gonadal sex. This result, observed by gross inspection of gonadal tissue and failure to observe successful fertilizations in the all-female communities, is in agreement with past work on this species (J. Rhodes and R. Fernald personal communication). Brooding was observed for both dominant and subordinate females. Of the 14 females that showed dominance behavior in the long-term observation communities, 11 (78%) had spawned at least once, whereas of the 25 females that displayed subordinate status, 13 (52%) had spawned. This difference is not significantly different (Fisher Exact test; p=0.171). All of the control females spawned at least once.
Territorial Tenure of Dominant Females
Among the three separate long-term communities, a total of 14 out of 29 individuals exhibited the male-typical dominant phenotype for at least some portion of the experiment, underscoring the dynamic social life of this species. Eleven of these 14 females lost their status at least once (three lost it twice; one lost it three times), and all but two of those later regained their status. In the majority of cases (69%) territory loss occurred shortly after spawning. Median duration of tenure as a dominant female was 3.6 weeks, for subordinates the median was 3.4 weeks. When we disregard the short periods (several days) of subordinate status immediately after brooding, median time spent as dominant was 3.9 weeks and median time as subordinate was 4.9 weeks.
Circulating Sex Steroid Hormones
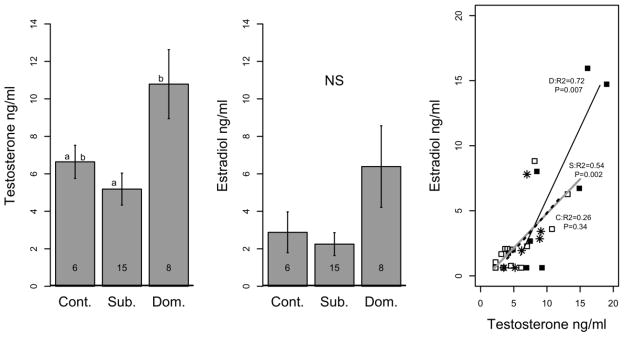
Blood samples for assaying hormone concentrations were obtained from eight dominant, fifteen subordinate, and six control females from the long-term communities. All of these fish had maintained social status at least four weeks prior to sampling. Circulating testosterone levels varied significantly among phenotypes (F2,26=5.85, p=0.0067) (DOM: 10.79±1.97, SUB: 5.19±0.88, Control: 6.64±0.97ng/mL). T levels were significantly higher in dominant compared to subordinate females (p=0.0087), but not compared to control females (p=0.0829; Figure 4A). When we measured circulating estradiol levels, the variation across the experimental groups was significant (F2,26=3.16, p=0.047), However, while pair wise comparisons were not significant after false discovery rate correction, estradiol levels showed the same pattern as testosterone in that dominant females exhibited higher titers than either subordinate (p=0.0612) or control females (p=0.1205) (DOM: 6.39±2.32, SUB: 2.25±0.63, Control: 2.88±1.19 ng/ml) (Figure 4B).
Figure 4. Hormone levels in female phenotypes.
Circulating levels (mean ± SE error) of testosterone (A) and estradiol (B) are shown. Numbers indicate sample sizes, letters indicate statistically significant differences (p < 0.05). (C) Correlation between steroid titers for individuals of each phenotype. Regression coefficients and p-values are given for D:dominant (black line), S:subordinate (gray line), and C:control (dashed line) female.
There was a strong positive relationship between testosterone and estradiol levels within dominant and subordinate phenotypes, but not in control females, as revealed by linear regression analysis (DOM: F1,6=15.79, R2=0.725, p=0.007; SUB: F1,13=15.47, R2=0.508, p=0.002; Control: F1,4=1.17, R2=0.226, p=0.341) (Figure 4C). While circulating estradiol did not correlate with any behavioral displays, we found that testosterone levels did tend to correlate with behavior patterns, (Table 2). Both chasing and threat behavior were positively correlated, while fleeing and digging behavior showed a negative correlation.
Table 2.
Spearman rho correlation coefficients (n=29) relating circulating steroid hormone levels and behavioral measures (averaged across week before blood collection). Uncorrected p-values are reported in parentheses, with values of p<0.05 highlighted in bold.
| Behavior | Hormone | |
|---|---|---|
| [Estradiol] | [Testosterone] | |
| Chase | 0.221 (0.248) | 0.377 (0.044) |
| Border Fight | 0.114 (0.556) | 0.306 (0.105) |
| Threat | 0.235 (0.219) | 0.403 (0.029) |
| Flee | −0.276 (0.146) | −0.375 (0.045) |
| Digging | −0.114 (0.556) | 0.195 (0.309) |
| School | −0.283 (0.136) | −0.437 (0.018) |
| Dom. Index | 0.204 (0.289) | 0.274 (0.015) |
Gonadosomatic Index
We calculated the gonadosomatic index (GSI) as a measure of reproductive status for a subset of the females from each phenotype in the short-term communities only (DOM: 7.5±2, n=4; SUB: 5.2±1.4, n=6; Control: 0.9±0.2, n=6). While there was significant variation among phenotypes (F2,13=6.748, p=0.0034), largely due to the low GSI of the control females relative to dominant (p=0.0081). Dominant and subordinate females did not differ in this measure (p=0.1419). This is not unexpected because the control females were brooding and therefore their ovaries would not be expected to contain any developed oocytes. There were no significant correlations between GSI and behavioral measures when only the dominant and subordinate females were considered.
Somatic Growth and Condition
In the long-term communities, weekly growth rates averaged 0.88% length across the subset of females monitored, with dominant females trending towards faster growth (DOM: 1.6%±0.1%, n=14; SUB: 0.99%±0.04%, n=20; Control: 0.70%±0.02%, n=9). However, this variation in growth rate with respect to social status was not significant (F2,40 = 2.965, p = 0.0589). Similarly, condition factor (CF) was calculated for a subset of the individuals included in the behavior analysis above (DOM: 3.40±0.109, n=14; SUB: −0.074±0.150, n=20; Control: −0.364±0.299, n=9). While, CF differed among social phenotypes (F2,40=3.404, p=0.043), pair wise comparisons were not significant after false discovery rate correction (data not shown).
Male Reintroduction
We reasoned that for females a dominant phenotype may convey an advantage by increasing her mating success when a male does become available. To test this hypothesis, we introduced a single dominant male to each of six all-female communities after the female social hierarchy had been stable for at least four weeks. Of the 13 females that had experienced dominance, only four spawned within the 30-day observation period following male introduction. Importantly, the duration of territorial tenure did not predict whether a dominant female spawned or not (Spearman correlation; rho=0.151, p=0.44). The non-spawning dominant females maintained an eye-bar, continued aggressive behavior toward other females, and were vigorously attacked by the introduced males (data not shown). Among the 15 females that never experienced dominance, eight spawned within the 30-day observation period, which is not different from the dominant females (χ2 test; p=0.2049). However, of the individuals that did spawn, the dominant females (n=4) mated with the reintroduced male within 5.75±3.09 days, whereas subordinate females (n=8) needed 16.12±2.57 days, a significant difference (t-test; p=0.036).
Discussion
In the present study we have shown that, for all-female communities of the highly social cichlid species A. burtoni, individuals can acquire a social dominance phenotype that closely resembles the well-known male-typical phenotype associated with territory maintenance and access to potential mates. In all-female situations, dominant females show behavioral, morphological, and hormonal changes typical of dominant males of this species. However, dominant social status in females did not correlate with growth rate or reproductive status, which eliminates some of the confounding physiological variables associated with the study of social status in males of this species. This paradigm thus provides a great opportunity to decipher the mechanisms that specifically underlie socially mediated behavioral plasticity.
In females, the dominant phenotype created by removal of males, included direct aggressive behavior such as chasing as well as stereotypical displays of aggression such as lateral displays and border threats. Interestingly, these females also showed male typical behaviors of digging (usually associated with bower construction) and courtship directed toward other females. Contrary to aggression in males, among females, most of the aggressive behavior observed was directed toward the subordinate females rather than other dominant females, reflecting the strong social hierarchy within the community. With regard to behavior, subordinate females provide a suitable comparison to subordinate males. Importantly, subordinate females, unlike subordinate males, do not show decreased GSI nor increased growth rate. Thus, the similar behavioral phenotypes of subordinate females and subordinate males can be compared in the absence of these confounding variables as is also the case for the dominant phenotypes of the two sexes.
Our behavioral observations demonstrate that female dominance, like male dominance, is a plastic and reversible phenotype. While not every individual attained dominant social status during our observations, there was a high degree of social volatility, similar to the situation in males (Hofmann et al., 1999). Among males, dominance is primarily determined by relative size within the social group (Hofmann et al., 1999). Similarly, in 4 out of the 5 all-female communities, the females that adopted the subordinate phenotype were on average smaller (as measured by mass or by standard length) at the beginning of the experiment than females that eventually established a stable dominant phenotype (data not shown). Because we measured size at time intervals independent of status changes, we cannot conclusively demonstrate this relationship between size and probability to achieve dominance in a given social interaction for the females in this study. However, given the difference in mean size and the similarity in growth rates between dominant and subordinate females, one would expect that females could retain dominant status longer than males. However, the duration of tenure in a particular social status for females is more similar to male tenure under a fluctuating environment (DOM: 3 weeks; SUB: 4 weeks) than under a stable environment (DOM: 9.5 weeks; SUB: 7 weeks)(Hofmann et al., 1999), suggesting that the social volatility observed in all-female communities may be at least in part caused by their reproductive cycle, including spawning and (abandoned) brooding events.
We found a clear association between social phenotype and circulating testosterone in females as had been seen in males (Parikh et al., 2006). As seen in previous studies, testosterone levels in females were overall considerably lower than those reported for either dominant or subordinate males (e.g., (Greenwood et al., 2008; Trainor and Hofmann, 2006). Nonetheless, the elevated testosterone levels, and correlated increases in estradiol, in dominant females suggest a role for these steroids in female aggression. The lack of strong correlations between circulating levels of steroid hormones and specific behavior patterns suggests that their effects are indirect and/or several other factors play important roles here as well. Either scenario would reduce the amount of variation explained by steroid hormone levels. It could also be that ongoing aggressive interactions – such as border threats – lead to acute fluctuations in androgen levels. We did not test this “challenge hypothesis” (Wingfield et al., 1990) in our all-female communities, however recent reports suggest that such a response does occur during maternal care (Renn et al., 2009) or during aggressive interactions in gravid females (Desjardins personal communication). Finally, it is possible that 11-KT, which we did not measure, is more tightly associated with social behavior than other steroids. However, this is unlikely as numerous studies have clearly shown that in A. burtoni (Kidd et al., 2010; Parikh et al., 2006) as well as other Haplochromine cichlids (Dijkstra et al., 2012) this fish-specific androgen is always tightly associated with testosterone at about 10-fold lower concentrations, independent of sex (e.g. Kidd et al., 2010)
The two color phenotypes, yellow (n=9) or blue (n=2), that we observed in dominant females are reminiscent of the color polyphenism typical of dominant males (Korzan et al., 2008), even though several dominant females (n=4) showed no obvious coloration. Some of the females displayed color as early as three days after all-female experimental communities were established. While the intensity of the color was somewhat variable, females do not appear to switch color morphs as readily as males (Korzan et al., 2008). While size clearly plays a role in determining social status, baseline hormone levels and/or color variation (cichlid: Dijkstra et al., 2009; bird: (Pryke, 2007) may be contributing factors. These two traits are known to be correlated with each other and in some cases with aggression levels in a wide range of animals (e.g. cichlid fish: Dijkstra et al., 2012; reptiles: Huyghe et al., 2009; birds: Muller and Eens, 2009; baboons Higham et al., 2008).
Dominant females do not appear to suppress the GSI and reproductive physiology of subordinate females, which is in strong contrast to the well-known situation in males (see, for example, Hofmann and Fernald, 2000). We found that both dominant and subordinate individuals in the all-female communities possessed ovaries of similar relative size (as measured by GSI) and continued to spawn and even incubate (unfertilized) eggs, thus demonstrating that both phenotypes produce mature ova. Similarly, dominant and subordinate females were in similar condition, whereas both GSI and condition were decreased in control females, which refrain from feeding during egg incubation (Renn et al., 2010). The observation that many dominant females transiently lost their social status immediately after spawning suggests that dominance behavior can nevertheless also be regulated by the reproductive cycle in addition to the social environment. The fact that the median tenure as dominant female (3.9 weeks) is almost exactly as long as the female reproductive cycle (Kidd et al., 2012) further suggests a role of reproductive physiology. While in many other teleost species changes in social status are often accompanied by sex change, our results are consistent with previous observations in cichlids of behavioral sex-role change without subsequent gonadal sex change in cichlids (reviewed by Oldfield, 2005).
It has been suggested that female aggression might be associated with competition for males and/or in response to male aggression at the lek (Karvonen et al., 2000; Desjardins personal communication). We therefore hypothesized that male-typical dominance behavior might enhance a female’s future spawning opportunities. However, the duration of territorial tenure did not predict whether a dominant female spawned or not. Subordinate females were just as likely as dominant individuals to spawn with an introduced male, although it took them almost three times as long to obtain a mating. Careful field studies will be needed to determine whether the male-typical dominance behavior we have described here indeed provides females with any adaptive advantage in the context of mate acquisition. Alternatively, the ultimate function of female dominance could derive from other social situations among the females. For example, female aggression and dominance status affect reproductive success in a wide variety of species and contexts (Heinsohn et al., 2005; Kinahan and Pillay, 2008; Sterck et al., 1997). Furthermore, recent evidence from a newly collected wild stock of A. burtoni suggests that maternal care after release of the fry from the buccal cavity is much more extensive than previously appreciated (Renn et al., 2009). Maternal care in this species likely involves territorial defense of a temporary nest site, as was first noted in field observations (Fernald and Hirata, 1977a). Clearly, more research is needed in both lab and field settings to further examine these and other adaptive explanations.
Conclusions
The novel experimental paradigm introduced here, which utilizes social hierarchies in all-female communities, provides a great opportunity to examine the physiological and molecular mechanisms underlying social dominance in a context that parallels the well-studied situation in males, yet differs in important aspects. We have shown that, in the absence of males, female A. burtoni can display male-typical social dominance behavior and associated circulating androgen levels reminiscent of dominant males. In contrast to the situation in males, dominant and subordinate females differ little in reproductive maturity or somatic growth. Thus, the all-female communities examined in the current study in conjunction with the well-established social plasticity in males provide an excellent opportunity to identify common molecular and physiological underpinnings of social dominance independent of sex.
Highlights.
In all-female communities of A. burtoni cichlids some individuals exhibit male-typical dominance behavior
Dominant and subordinate females differ in social behavior and circulating sex steroid hormones
Potentially confounding factors such as gonadal state and growth do not vary
This paradigm provides a novel approach to the study of the mechanisms of social behavior.
Acknowledgments
We are grateful to Josiah Altschuler, Melinda Snitow and Jasmine Dowell for animal care. Lauren O’Connell for photography, and Heather Machado, Julia Carleton, Lauren O’Connell and Ronald Oldfield for comments on earlier versions of this manuscript. This research was supported by an NRSA post-doctoral fellowship to S.C.P.R.; the Harvard College Research Program to E.J.F.; a postdoctoral fellowship from FQRNT (Fonds Québécois de la Recherche sur la Nature et les Technologies) and a postdoctoral fellowship from the Natural Science and Engineering Research Council of Canada (NSERC) to N.A.H.; and by National Institutes of Health grant NIGMS GM068763 and the Bauer Center for Genomics Research (H.A.H.).
Footnotes
Authors Contributions
H.A.H, S.C.P.R. conceived the experiment. S.C.P.R. and E.J.F. conducted the behavioral observations. B.C.T. conducted the hormone assays. E.J.F., S.C.P.R, N.A.-H. and H.A.H. wrote the paper. All authors have read and approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnott G, Elwood R. Probing aggressive motivation in a cichlid fish. Biology Letters. 2009;5:762–764. doi: 10.1098/rsbl.2009.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin-Horth N, Desjardins JK, Martei YM, Balshine S, Hofmann HA. Masculinized dominant females in a cooperatively breeding species Mol. Ecol. 2007;16:1349–1358. doi: 10.1111/j.1365-294X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57:289–300. [Google Scholar]
- Burmeister SS. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Hazelden MR, Van der Kraak GJ, Balshine S. Male and female cooperatively breeding fish provide support for the “Challenge Hypothesis”. Behav Ecol. 2006;17:149–154. [Google Scholar]
- Dijkstra PD, Verzijden MN, Groothuis TGG, Hofmann HA. Divergent hormonal responses to social competition in closely related species of Haplochromine cichlid fish. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.01.011. in revision. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Anim Behav. 1977;25:643–653. [Google Scholar]
- Fernald RD. Social influences on the brain. Horm Behav. 2004;46:129–130. [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni : Quantitative behavioral observations. Anim Behav. 1977a;25:964–975. [Google Scholar]
- Fernald RD, Hirata NR. Field Study of Haplochromis burtoni: Habitats and co-habitants. Environ Biol Fishes. 1977b;2:299–308. [Google Scholar]
- Fernald RD, Liebman PA. Visual Receptor Pigments in the African Cichlid Fish, Haplochromis-Burtoni. Vision Res. 1980;20:857–864. doi: 10.1016/0042-6989(80)90066-8. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc R Soc B-Biological Sciences. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsohn R, Legge S, Endler JA. Extreme reversed sexual dichromatism in a bird without sex role reversal. Science. 2005;309:617–619. doi: 10.1126/science.1112774. [DOI] [PubMed] [Google Scholar]
- Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. Baboon sexual swellings: Information content of size and color. Horm Behav. 2008;53:452–462. doi: 10.1016/j.yhbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Winkler H, Oliveira RF. Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm Behav. 2003;43:508–519. doi: 10.1016/s0018-506x(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Hofmann H. Functional genomics of neural and behavioral plasticity. J Neurobiol. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Fernald RD. Social status controls somatostatin neuron size and growth. J Neurosci. 2000;20:4740–4744. doi: 10.1523/JNEUROSCI.20-12-04740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: Consequences for life-history strategies. Proc Natl Acad Sci U S A. 1999;96:14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe K, Husak JF, Herrel A, Tadic Z, Moore IT, Van Damme R, Vanhooydonck B. Relationships between hormones, physiological performance and immunocompetence in a color-polymorphic lizard species, Podarcis melisellensis. Horm Behav. 2009;55:488–494. doi: 10.1016/j.yhbeh.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The Androgen Receptor Governs the Execution, but Not Programming, of Male Sexual and Territorial Behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen E, Rintamaki PT, Alatalo RV. Female-female aggression and female mate choice on black grouse leks. Anim Behav. 2000;59:981–987. doi: 10.1006/anbe.1999.1379. [DOI] [PubMed] [Google Scholar]
- Kidd CE, Kidd MR, Hofmann HA. Measuring multiple hormones from a single water sample using enzyme immunoassays. Gen Comp Endocrinol. 2010;165:277–285. doi: 10.1016/j.ygcen.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Kidd MR, O’Connell LA, Kidd CE, Chen CW, Fontenot MR, Sepeda D, Williams SJ, Hofmann HA. Prostaglandin F2α mediates female mate choice in an African cichlid. Proc R Soc B. 2012 in revision. [Google Scholar]
- Kinahan AA, Pillay N. Dominance status influences female reproductive strategy in a territorial African rodent Rhabdomys pumilio. Behavioral Ecology and Sociobiology. 2008;62:579–587. [Google Scholar]
- Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the plainfin midshipman fish (Porichthys notatus) Horm Behav. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Robison RR, Zhao S, Fernald RD. Color change as a potential behavioral strategy. Horm Behav. 2008;54:463–470. doi: 10.1016/j.yhbeh.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Behavioral and physiological plasticity: Rapid changes during social ascent in an African cichlid fish. Horm Behav. 2010;58:230–240. doi: 10.1016/j.yhbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21:353–363. [PubMed] [Google Scholar]
- McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Philos Trans R Soc Lond Ser B-Biol Sci. 2008;363:1611–1620. doi: 10.1098/rstb.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W, Eens M. Elevated yolk androgen levels and the expression of multiple sexually selected male characters. Horm Behav. 2009;55:175–181. doi: 10.1016/j.yhbeh.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Hormones and Social Behavior. 3. Chapter 8. Sinauer: Sunderland (MA); 2005. An Introduction to Behavioral Endocrinology. [Google Scholar]
- O’Connell LA, Hofmann HA. Levels of Biological Organization. 2011. Social Status Predicts how Sex Steroid Receptors Regulate Complex Behavior across. (submitted) [DOI] [PubMed] [Google Scholar]
- Oldfield RG. Genetic, abiotic and social influences on sex differentiation in cichlid fishes and the evolution of sequential hermaphroditism. Fish and Fisheries. 2005;6:93–110. [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM. Social modulation of androgen levels in male teleost fish. Comp Biochem and Phys B. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav Brain Res. 2006;166:291–295. doi: 10.1016/j.bbr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Pryke SR. Fiery red heads: female dominance among head color morphs in the Gouldian finch. Behav Ecol. 2007;18:621–627. [Google Scholar]
- Renn SCP, Aubin-Horth N, Hofmann HA. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 2008;211:3041–3056. doi: 10.1242/jeb.018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Carleton JB, Magee H, Nguyen MLT, Tanner ACW. Maternal care and altered social phenotype in a recently collected stock of Astatotilapia burtoni cichlid fish. Int Comp Biol. 2009;49:660–673. doi: 10.1093/icb/icp085. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Fernald RD, Clayton DF. Genes and Social Behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Amurumarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and Nonneuronal Aromatase in Primary Cultures of Developing Zebra Finch Telencephalon. J Neurosci. 1994;14:7541–7552. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45:225–234. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Sterck EHM, Watts DP, vanSchaik CP. The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol. 1997;41:291–309. [Google Scholar]
- Trainor BC, Hofmann HA. Somatostatin regulates aggressive behavior in an African cichlid fish. Endocrinology. 2006;147:5119–5125. doi: 10.1210/en.2006-0511. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Fernald RD. Gonadotropin-Releasing-Hormone Containing Neurons Change Size with Reproductive State in Female Haplochromis-Burtoni. J Neurosci. 1993;13:434–441. doi: 10.1523/JNEUROSCI.13-02-00434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J Exp Biol. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The Challenge Hypothesis - Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Wong RY, Hofmann HA. Encyclopedia of Life Sciences. John Wiley & Sons Ltd; Chichester: 2010. Behavioural Genomics: An Organismic Perspective. [Google Scholar]