Abstract
Context:
Endocannabinoid receptor 1 blockade is proposed to improve metabolic complications of obesity via central and peripheral effects.
Objective:
Our objective was to test whether rimonabant improves insulin regulation of free fatty acid and glucose metabolism after controlling for fat loss.
Design:
This was a double-blind, placebo-controlled substudy of the visceral fat reduction assessed by computed tomography scan on rimonabant (VICTORIA) trial.
Participants and Setting:
Sixty-seven abdominally obese, metabolic syndrome volunteers age 35–70 yr participated at academic medical center general clinical research centers.
Intervention:
Intervention included a 12-month lifestyle weight management program plus rimonabant 20 mg/d or placebo.
Main Outcome Measures:
Body composition and two-step euglycemic, hyperinsulinemic clamp before and after intervention were performed. Insulin sensitivity was assessed as insulin concentration needed to suppress by 50% palmitate concentration [IC50(palmitate)], flux [IC50(palmitatef], and hepatic glucose output [IC50(HGO)] and as insulin-stimulated glucose disposal (Δ glucose disappearance per Δ insulin concentration − glucose slope).
Results:
Body fat decreased by 4.5 ± 2.9% (SD) in the rimonabant and 1.9 ± 4.5% in the placebo group (P < 0.005). The primary [improvement in IC50(palmitate) and IC50(palmitate)f] and secondary [improvement in IC50(HGO) and glucose slope] outcomes were not significantly different between the rimonabant and placebo groups. Post hoc analyses revealed that 1) changes in body mass index (BMI) and IC50(palmitate) were correlated (P = 0.005) in the rimonabant group; this relationship was not significantly different from placebo when controlling for greater BMI loss (P = 0.5); 2) insulin-regulated glucose disposal improved in both groups (P = 0.002) and correlated with changes in BMI.
Conclusions:
Improvements observed in insulin regulation of free fatty acid and glucose metabolism with rimonabant treatment in humans was not greater than that predicted by weight loss alone.
There are two endocannabinoid system receptor subclasses, of which cannabinoid 1 receptors (CB1R) are important in regulating ingestive behavior. First identified within the central nervous system, CB1R are found in numerous peripheral tissues, including adipocytes, muscle, and liver (1). Rimonabant was the first CB1R antagonist developed for weight reduction therapy. It was briefly available for clinical use in Europe before being withdrawn over concerns regarding adverse psychiatric events (e.g. depression and suicidal ideation). Interest in CB1R antagonists remains because data from in vitro and animal studies suggest they may have independent, beneficial effects on glucose and lipid metabolism in peripheral tissues as well as stimulating greater energy expenditure (1–8). Indirect support for this concept has come from large-scale clinical trials [Rimonabant in Obesity (RIO)-North America (9), RIO-Europe (10), RIO-Lipids (9, 11), and RIO-Diabetes (12)]. However, previous studies have not directly measured the impact of CB1R antagonism on peripheral glucose and free fatty acid (FFA) in humans controlling for the effects of weight loss.
The goal of this study was to quantify the in vivo effects of rimonabant on glucose and fatty acid metabolism compared with placebo as a substudy of the visceral fat reduction assessed by computed tomography (CT) scan on rimonabant (VICTORIA) trial.
Subjects and Methods
Study aims and population
Main study group
The VICTORIA study was a double-blinded two-arm and placebo-controlled trial designed to measure the effect of rimonabant on visceral fat in abdominally obese adults with features of the metabolic syndrome as defined at the time of protocol development. There were 27 international study sites with data collection between February 2006 and July 2008 (inclusion and exclusion criteria are provided in Table 1). The primary aim was to compare changes in visceral fat and metabolic syndrome components in volunteers treated with lifestyle intervention (diet, activity, and behavior therapy) for 12 months and randomized to rimonabant 20 mg daily vs. placebo. Secondary aims of the main VICTORIA study were to assess changes in anthropometric measures, lipids and lipoprotein profiles, glycemia and insulinemia, and adipokine, inflammatory, and hemostatic markers. Participants were randomized for equal numbers of men and women in each group.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Waist circumference >102 cm men, >88 cm women | Weight management history of Significant weight loss or very-low-calorie diet (≤800 kcal/d) within 3 months of screening visit |
| Age 35–70 yr | |
| At least two other components of the metabolic syndrome as defined by the NCEP/ATPIII classification: | Previous bariatric surgery |
| Triglyceridemia ≥150 mg/dl (or 1.69 mmol/liter) | Weight reduction medication |
| HDL cholesterol <50 mg/dl (1.29 mmol/liter) in women or <40 mg/dl (1.04 mmol/liter) in men | Presence of eating disorders |
| Blood pressure ≥130/85 mm Hg (systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg) or treatment with antihypertensive agent(s) for this condition | Endocrine |
| Fasting blood glucose >110 mg/dl (or 6.1 mmol/liter) | Presence of diabetes |
| Any clinically significant endocrine disorder | |
| Any organ dysfunction | |
| Lipid-lowering agents (HMG CoA reductase inhibitors allowed if dose stable for 3 months) | |
| Prolonged use (>10 d) of corticosteroids and neuroleptics | |
| Cancer diagnosis in the preceding 5 yr | |
| Psychological | |
| Any episode severe depression | |
| >2 episodes previous depression | |
| Use of antidepressants | |
| Pregnancy and breastfeeding |
HMG CoA, 3-hydroxy-3-methyl-glutaryl coenzyme A; NCEP/ATP, National Cholesterol Education Program/Adult Treatment Panel.
Substudy
Four centers participated in a substudy with the primary aim to understand whether changes in the insulin regulation of FFA and glucose metabolism are different between those receiving rimonabant vs. placebo combined with a weight loss intervention. Those four centers enrolled 67 volunteers, one of whom did not complete the first insulin clamp study, leaving 66 volunteers with baseline data.
Lifestyle intervention, sample collection, and adverse event monitoring were the same as for the main study group. Participants in the substudy additionally underwent indirect calorimetry, two-step euglycemic-hyperinsulinemic clamp and tracer studies of glucose and FFA metabolism before and after 12 months of treatment. Only results from the substudy participants are reported herein.
Methods
Body composition
Height, weight, and waist circumference were recorded at each study visit. At baseline and 12 months, total and regional body fat was measured using dual-energy x-ray absorptiometry and visceral and abdominal sc fat areas (square centimeters) were measured from a single-slice abdominal CT at the L4-L5 interspace. Analysis of CT images was centralized and performed in conjunction with quality control images to ensure consistency.
Diet
Energy requirements were based on calculated basal metabolic rates and physical activity levels. A mild hypocaloric diet (600 kcal/d deficit) was prescribed, but keeping energy intake at 1200 kcal/d or higher for women and 1600 kcal/d or higher for men. Macronutrient distribution was recommended to be approximately 50% carbohydrate, 30% fat, and 20% protein with alcohol consumption below 15 g/d or below 85 g/wk. Participants also received instructions for physical activity, taking into account their medical condition (e.g. walking 30 min above the daily walking habits). Dietary and activity review were performed at each visit.
Blood samples
Blood was collected under overnight postabsorptive conditions for measurement of plasma glucose, insulin (mean of three samples), FFA-palmitate (mean of four samples), and other tests of metabolic and inflammatory parameters including hemoglobin A1c (HbA1c) lipid profile, and adipokines. Alcohol consumption within 48 h and intense physical activity within 24 h preceding blood sampling were discouraged. Plasma glucose concentrations were measured with a glucose analyzer (Beckman Instruments, Fullerton, CA), and plasma insulin by chemiluminescent sandwich assays (Sanofi Diagnostics, Chaska, MN). Plasma triglyceride concentrations were measured using microfluorometric assay (13), FFA-palmitate concentrations and enrichment by liquid chromatography/mass spectrometry (14), and glucose enrichment using gas chromatography/mass spectrometry (15).
Euglycemic-hyperinsulinemic clamp
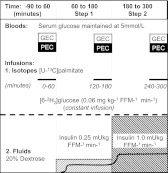
A two-step euglycemic-hyperinsulinemic clamp study (Fig. 1) was used to establish 1) insulin regulation of glucose metabolism as estimated by the insulin concentration needed to suppress hepatic glucose output (HGO) by 50% [IC50(HGO)] and glucose disposal slope [the change in glucose disposal in milligrams per kilogram fat-free mass (FFM) per minute per unit change in insulin concentration] and 2) FFA insulin sensitivity as estimated by the insulin concentration needed to suppress palmitate concentrations and flux by 50% [IC50(palmitate) and IC50(palmitate)f, respectively) and basal lipolysis. An iv catheter was placed for infusions of recombinant human insulin at 0.25 mU/kg FFM · min (step 1) and 1.0 mU/kg FFM · min (step 2), [U-13C]palmitate (Cambridge Isotope Laboratories, Andover, MA), and [6-2H2]glucose (Isotec Inc., Miamisburg, OH) (Fig. 2). A second retrograde catheter was placed into a dorsal hand vein to collect arterialized blood using the heated box technique. Glucose disposal was calculated after reaching steady state using linear regression modeling.
Fig. 1.
Schematic of euglycemic-hyperinsulinemic clamp study. Isotopes administered were 1) [6-2H2]glucose (constant infusion), 2) [U-13C]palmitate (infused over the last 60 min of each study interval), and 3) 20% dextrose labeled with [6-2H2]glucose (to maintain euglycemia at 5 mmol/liter). Blood sampling for glucose enrichment and concentration (GEC) and palmitate enrichment and concentration (PEC) was performed over the last 30 min of each time period.
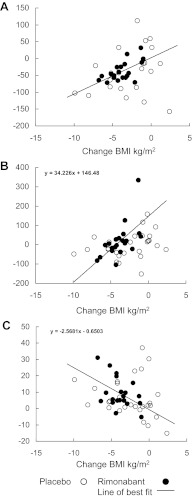
Fig. 2.
Correlations between changes in BMI and insulin sensitivity of glucose and fatty acid metabolism are shown. In the rimonabant group, there were significant correlations between changes in BMI and changes in insulin sensitivities of palmitate concentration (r = 0.61; P = 0.004) (A), hepatic glucose production (HGO) (r = 0.61; P = 0.003) (B), and glucose disposal (r = 0.45; P = 0.05) (C). Although the trends were not significant in the placebo group, the slopes of association were not different.
Indirect calorimetry
Indirect calorimetry was performed with a ventilated hood during the last 30 min of the basal period, and the low-dose and high-dose hyperinsulinemic periods and resting energy expenditure and respiratory exchange ratios (RER) were calculated.
Clinical safety
Patients were seen at months 1, 3, 5, 7, 9, and 12 for assessment of dietary compliance and dispensation of study medications and contacted by phone at months 2, 4, 6, 8, 10, and 11. There was monthly assessment of study drug compliance, concomitant medications, adverse events, and neuropsychiatric scripted questions. Physical examination and standard hematology and serum chemistry were performed at screening and months 3, 7, and 12 or at permanent premature study discontinuation.
Statistical analysis
Power calculations for the substudy were based upon the goal of detecting a difference between the rimonabant and placebo groups in the ability of insulin to enhance suppression of FFA after weight loss, which was the primary, prespecified end point of the substudy. We used previously published data (16) to estimate the mean and sd of palmitate concentrations and kinetics under basal and insulin-suppressed conditions in obese, nondiabetic, and insulin-resistant patients. We estimated that a 30% improvement in insulin suppression of palmitate would be a clinically significant improvement after active treatment. Given a sd of 36%, an α-level of 5% with a power of 80% and for a two-sided t test, the required sample size to detect a 30% difference between groups was 24 patients per arm. Within-group differences were compared using repeated-measures ANOVA. Palmitate and insulin relationships were analyzed using linear regression approaches after log transformation of both values to examine the dose-response relationships (17). Secondary, prespecified end points include between-group comparisons of changes in 1) the ability of insulin to stimulate glucose disposal (change in glucose slope) and suppress hepatic glucose production [change in IC50(HGO)]; 2) markers of metabolic health (adiponectin and fibrinogen), serum triglycerides and total cholesterol, apolipoprotein B (Apo B) concentrations, inflammatory markers (high-sensitivity C-reactive protein, plasminogen activator inhibitor-1, and TNF-α). The remainder of the analyses we present (relationships between FFA and glucose variables and relationships between body composition variables and metabolic variables) represent post hoc analysis designed to explore associations that might relate to the heterogeneity in the data. Data are provided as mean ± sd, and statistical significance was analyzed using ANOVA and t tests for normally distributed data and Wilcoxon signed rank test for nonparametric data. Nonparametric data were log transformed if needed to allow for standard regression approaches. Correlation coefficients (r) are provided as indicators of relationship strength. Variate analyses and standard least-squares regression analyses were performed using JMP statistical software.
Results
VICTORIA study participant characteristics
The VICTORIA parent study included 254 participants. The demographic and baseline characteristics were similar in the placebo and rimonabant treatment arms, and the proportion of metabolic syndrome defining criteria was equal in the two groups.
VICTORIA substudy baseline subject characteristics (Table 2)
Table 2.
VICTORIA substudy baseline subject characteristics
| Baseline characteristic | All baseline |
Completed |
P value between complete groups | ||
|---|---|---|---|---|---|
| Placebo | Rimonabant | Placebo | Rimonabant | ||
| Number | 35 | 31 | 27 | 21 | |
| Age (yr) | 54 ± 7 | 55 ± 9 | 54 ± 8 | 56 ± 9 | 0.35 |
| Male/female | 13/22 | 12/20 | 8/17 | 8/12 | |
| BMI (kg/m2) | 35.2 ± 4.1 | 33.5 ± 3.1 | 34.9 ± 4.2 | 32.8 ± 2.4 | 0.18 |
| Total body weight (kg) | 102.5 ± 18.6 | 98.2 ± 15.5 | 100.0 ± 17.7 | 97.6 ± 14.0 | 0.70 |
| Waist circumference (cm) | 114 ± 12 | 110 ± 9 | 113 ± 11 | 108 ± 8 | 0.45 |
| Percent body fat | 43 ± 8 | 44 ± 9 | 44 ± 2 | 43 ± 10 | 0.82 |
| Total body fat (kg) | 43.0 ± 8.7 | 41.2 ± 8.6 | 42.6 ± 1.7 | 40.6 ± 8.6 | 0.42 |
| Visceral fat area (cm2) | 193 (160–253) | 184 (165–206) | 191 (159–250) | 184 (169–198) | 0.67 |
| sc fat area (cm2) | 458 ± 104 | 415 ± 89 | 466 ± 104 | 407 ± 79 | 0.05 |
| Deep sc fat area (cm2) | 260 ± 83 | 227 ± 73 | 256 ± 78 | 226 ± 63 | 0.13 |
| Fasting blood glucose (mg/dl) | 107 ± 13 | 105 ± 12 | 104 ± 11 | 104 ± 13 | 0.93 |
| Glucose slope (×1000) (mg · kg FFM−1 · min−1/pmol · liter−1) | 7.4 (18.0–13.8) | 12.0 (6.8–16.7) | 8.5 (1.4–14.2) | 13.2 (6.4–20.6) | 0.26 |
| IC50 hepatic glucose suppression (pmol/liter) | 229 (144–257) | 159 (121–208) | 207 (134–263) (n = 26) | 149 (121–197) (n = 21) | 0.05 |
| IC50(palmitate)f (pmol/liter) | 183 (122–249) | 129 (111–158) | 183 (118–243) (n = 20) | 140 (116–167) (n = 19) | 0.05 |
| IC50(palmitate) (pmol/liter) | 152 (104–209) | 113 (99–150) | 148 (92–202) (n = 22) | 115 (95–151) (n = 20) | 0.13 |
| Baseline RER | 0.77 ± 0.06 | 0.78 ± 0.06 | 0.76 ± 0.06 | 0.78 ± 0.06 | 0.33 |
| Low insulin rate RER | 0.78 ± 0.05 | 0.79 ± 0.05 | 0.77 ± 0.05 | 0.79 ± 0.06 | 0.12 |
| High insulin rate RER | 0.82 ± 0.06 | 0.84 ± 0.07 | 0.82 ± 0.06 | 0.85 ± 0.07 | 0.16 |
| BMR (kcal/24 h) | 2747 ± 523 | 2616 ± 538 | 2715 ± 539 | 2591 ± 489 | 0.27 |
| Adiponectin (μ g/ml) | 5.13 (4.14–6.51) | 6.54 (3.97–7.45) | 5.43 (4.29–6.03) | 6.5 (3.85–7.31) | 0.69 |
| Fibrinogen (g/liter) | 3.0 ± 0.8 | 3.0 ± 0.7 | 2.9 ± 0.9 | 3.0 ± 0.6 | 0.60 |
| Leptin (ng/ml) | 32.1 (20.8–54.0) | 29.4 (18.6–61.9) | 36.9 (20.8–62.4) | 29.5 (18.3–61.0) | 0.76 |
| Apo B (g/liter) | 0.96 ± 0.29 | 0.97 ± 0.25 | 0.99 ± 0.27 | 0.99 ± 0.26 | 0.94 |
| HDL cholesterol (mmol/liter) | 1.09 (1.01–1.23) | 1.13 (1.03–1.35) | 1.14 (1.04–1.4) | 1.17 (1.07–1.36) | 0.24 |
| LDL cholesterol (mmol/liter) | 3.27 ± 0.90 | 3.56 ± 0.86 | 3.29 ± 0.87 | 3.62 ± 0.96 | 0.26 |
| Total cholesterol (mmol/liter) | 5.30 ± 1.06 | 5.75 ± 0.91 | 5.39 ± 1.00 | 5.85 ± 0.97 | 0.16 |
| Triglycerides (mmol/liter) | 1.83 (1.08–1.83) | 1.97 (1.72–2.26) | 1.89 (1.44–2.15) | 2.00 (1.72–2.26) | 0.20 |
| HbA1c (%) | 5.7 (5.5–5.9) | 5.6 (5.4–5.8) | 5.7 (5.5–5.9) | 5.6 (5.3–5.8) | 0.19 |
Data that are normally distributed are presented as mean ± sd, and data that are not normally distributed are presented as median (interquartile range). Data for all study completers n = 48 unless otherwise stated. HDL, High-density lipoprotein; LDL, low-density lipoprotein.
Sixty-seven participants were randomized, of which 48 reached study completion (27 placebo and 21 rimonabant). There were no statistically significant baseline differences between the two groups of study completers for any of the parameters.
For the entire group studied before randomization, we found the expected relationships between baseline IC50(palmitate)f and palmitate concentration insulin sensitivity [IC50(palmitate)] (r = 0.74; P < 0.001). Likewise, baseline indices of insulin-regulated FFA and glucose metabolism were interrelated: IC50(palmitate)f and IC50(HGO) were well correlated (r = 0.66; P < 0.001), as were IC50(palmitate)f and the insulin/glucose disposal slope (r = −0.40; P < 0.001). IC50(palmitate) was also correlated with IC50(HGO) (r = 0.84; P < 0.001) and glucose slope (r = −0.53; P < 0.001).
Deep sc fat area was correlated with IC50(palmitate)f (r = 0.48; P < 0.001) and IC50(palmitate) (r = 0.35; P = 0.003). Visceral fat area was correlated with glucose disposal (r = −0.34; P = 0.005), IC50(HGO) (r = 0.37; P = 0.002), and IC50(palmitate) (r = 0.33; P = 0.007). Of note in making inferences for these relationships, deep sc and visceral fat areas in these participant were correlated with each other (r = 0.26; P = 0.03), but total sc and visceral fat areas were not significantly correlated.
Basal metabolic rate (BMR) was strongly correlated with FFM (r = 0.77; P < 0.001), suggesting to us that the dual-energy x-ray absorptiometry instruments and indirect calorimeters from each site provided comparable data.
Response to interventions (Table 2)
Body composition changes
Waist circumference and body mass index (BMI) decreased significantly in both groups at 1 yr, with greater reductions in the rimonabant group than the placebo group (Table 3). Reductions in abdominal deep sc adipose tissue and visceral fat compartments by CT were significant and correlated with overall weight loss, but not treatment type.
Table 3.
Comparison of change from baseline data for placebo and rimonabant groups
| Parameter | n | Placebo group mean change ± sd | Rimonabant group mean change ± sd | P value between group mean changes |
|---|---|---|---|---|
| Body composition parameters | ||||
| Weight change (kg) | 48 | −7.4 ± 9.4a | −11.0 ± 4.4a | <0.05 |
| Waist circumference change (cm) | 47 | −9 ± 8a | −11 ± 7a | 0.18 |
| BMI change (kg/m2) | 48 | −2.5 ± 3.0a | −3.8 ± 1.6a | <0.05 |
| Fat mass change (kg) | 48 | −4.6 ± 7.4b | −8.3 ± 3.4a | <0.01 |
| % body fat change | 48 | −1.9 ± 4.5d | −4.5 ± 2.9a | <0.005 |
| FFM change (kg) | 48 | −3.0 ± 3.8a | −2.3 ± 2.3b | 0.51 |
| Visceral fat area change (cm2) | 47 | −42 ± 52a | −53 ± 30a | 0.11 |
| sc fat area change (cm2) | 42 | −51 ± 66a | −82 ± 52a | <0.05 |
| Deep sc fat area change (cm2) | 46 | −20 ± 49d | −40 ± 28a | <0.05 |
| FFA and glucose metabolism | ||||
| IC50(palmitate)f change (pmol/liter) | 39 | −29 ± 73b | −69 ± 75c | 0.21 |
| Glucose slope change (×1000) (mg · kg FFM−1 · min−1/pmol · liter−1) | 48 | 6 ± 12b | 9 ± 9a | 0.10 |
| IC50(HGO) change (pmol/liter) | 47 | −4 ± 67 | 16 ± 89 | 0.44 |
| IC50(palmitate) change (pmol/liter) | 42 | −40 ± 68b | −38 ± 23a | 0.94 |
| BMR (kcal/24 h) | 48 | −225 ± 354 | −193 ± 266b | 0.38 |
| Low insulin rate RER | 47 | 0.045 ± 0.28 | 0.002 ± 0.11 | 0.93 |
| High insulin rate RER | 48 | 0.003 ± 0.09 | −0.035 ± 0.11 | 0.23 |
| Serum markers | ||||
| Adiponectin (μg/ml) | 47 | 3.13 ± 2.83a | 3.71 ± 3.05a | 0.64 |
| Fibrinogen (g/liter) | 47 | 0.7 ± 0.9a | 0.9 ± 0.7a | 0.77 |
| Leptin (ng/ml) | 47 | −13.5 ± 15.0a | −14.0 ± 13.1a | 0.57 |
| Apo B (g/liter) | 47 | −0.1 ± 0.2 | 0.1 ± 0.3 | <0.05 |
| HDL cholesterol (mmol/liter) | 47 | −0.05 ± 0.20 | −0.04 ± 0.2 | 0.74 |
| LDL cholesterol (mmol/liter) | 47 | −0.5 ± 0.60a | −0.2 ± 1.0 | 0.16 |
| Total cholesterol (mmol/liter) | 47 | −0.8 ± 0.80a | −0.6 ± 1.0d | 0.53 |
| Triglycerides (mmol/liter) | 47 | −0.4 ± 0.5a | −0.7 ± 0.8a | 0.22 |
| HbA1c (%) | 43 | −0.2 ± 0.3b | −0.2 ± 0.2a | 0.71 |
Significance of difference between baseline and end changes within each group is indicated after sd. HDL, High-density lipoprotein; LDL, low-density lipoprotein.
P < 0.001.
P < 0.005.
P < 0.01.
P < 0.05.
Glucose metabolism [glucose slope and IC50(HGO)]
Although in the nondiabetic range, HbA1c (n = 43) decreased significantly in both placebo and rimonabant groups (Table 3). One of the prespecified secondary outcome variables, insulin-regulated glucose disposal, as measured by the glucose slope, improved in both groups (Table 3). This improvement was greater, but not statistically significantly so, in the rimonabant group. Because of the heterogeneity in the glucose slope responses to the interventions, we examined post hoc whether the inter-individual changes correlated with changes in weight or body fat compartments. Changes in BMI correlated with changes in glucose slope in the rimonabant group (r = 0.04; P = 0.05) but not placebo group (P = 0.32), whereas we found no significant correlations between changes in glucose slope and changes in CT measured visceral or sc fat areas in either group.
There were no statistically significant changes in IC50(HGO) in either group; however, we also note significant heterogeneity in this response and also tested post hoc whether intra-individual changes in IC50(HGO) were related to variations in weight or regional fat loss. The changes in IC50(HGO) were correlated with changes in BMI (r = 0.61; P = 0.003) in the rimonabant group, and although there was a similar trend in the placebo group, the relationship was not statistically significant. There were no statistically significant correlations between IC50(HGO) change and visceral fat area change in either group.
FFA metabolism [IC50(palmitate)f and IC50(palmitate)]
There were significant decreases (improvements) in the primary, prespecified outcome variables IC50(palmitate)f and IC50(palmitate) in both groups (Table 3). Post hoc analysis revealed that IC50(palmitate) change was correlated with BMI change (r = 0.60; P = 0.004) in the rimonabant group. Although this correlation was not statistically significant in the placebo group, both study arms followed the same trend, and regression analysis did not reveal a significant effect of treatment arm on the relationship.
Glucose/FFA interrelationships
The changes in glucose slope were correlated with changes in insulin regulation of FFA in the placebo group (P = 0.03) but not in the rimonabant group (P = 0.8). In the placebo group, changes in IC50(HGO) were correlated with changes in IC50(palmitate) (r = 0.60; P < 0.001) and IC50(palmitate)f (r = 0.50; P = 0.008). The same trend was observed in the rimonabant group, where the change in IC50(HGO) correlated with the IC50(palmitate) change (r = 0.65; P = 0.001).
Energy metabolism
BMR decreased significantly within each group (P < 0.005), but the change was not significantly different between groups. After treatment, fasting RER did not change from baseline, pretreatment values.
Serum marker and lipid data
Both groups showed improvements in markers of metabolic health (adiponectin and fibrinogen, P < 0.001) and serum triglycerides and total cholesterol. Rimonabant use was associated with significant improvements in Apo B concentrations, which were statistically accounted for by the greater weight loss (Table 3). The inflammatory markers high-sensitivity C-reactive protein, plasminogen activator inhibitor-1, and TNF-α did not change significantly from baseline in either group (data not shown).
Adverse events and study dropouts
Adverse events were considered for all randomized substudy participants (placebo n = 35; rimonabant n = 31). Adverse events directly related to the study were recognized in 57% of the placebo arm (n = 20) and 74% of the rimonabant arm (n = 23). Five participants (16%) withdrew from the rimonabant group within 2 months after psychological events.
Psychological problems were the most common, affecting 57% of the placebo group (n = 20) and 61% of the rimonabant group (n = 19). Symptoms most commonly described were depression (placebo 31%, n = 11; rimonabant 35%, n = 11), anxiety disorder (placebo 17%, n = 6; rimonabant 29%, n = 9), loss of energy or concentration (placebo 11%, n = 4; rimonabant 16%, n = 5), and sleep disorder (placebo 29%, n = 10; rimonabant 26%, n = 8). Gastrointestinal side effects were also commonly reported, affecting 14% of the placebo group (n = 5) and 39% of the rimonabant group (n = 12). Reasons for noninclusion in the final data analysis included study dropout before completion resulting from adverse events and insufficient data collection (as indicated in Table 2).
Discussion
Clarifying whether CB1R antagonism has major peripheral actions has important clinical implications should agents that do not penetrate the central nervous system become available. Animal models support a role of CB1R on peripheral metabolism and energy expenditure, whereas it has been inferred from clinical studies that up to half of the beneficial lipid (RIO-Lipids) (11) and glycemic improvements (RIO-Diabetes) (12) seen with rimonabant treatment are independent of weight loss. This is the first clinical study to directly assess the effects of weight loss generated by rimonabant treatment vs. placebo on the insulin regulation of adipose tissue lipolysis and glucose metabolism. Although the rimonabant treatment group lost significantly more weight (11 ± 4%) than their placebo counterparts (7 ± 9%), improvements in glucose disposal, hepatic glucose production, and fatty acid metabolism were not significantly different between groups even without accounting for the differences in weight loss. Regression analysis, controlling for the amount of weight loss, indicated that improvements in metabolic outcomes were not different between the two groups. This suggests that any direct peripheral effects of rimonabant are minimal compared with the indirect benefits resulting from weight loss.
In these volunteers studied before and after weight loss, the improvements in insulin sensitivity with respect to glucose metabolism were strongly related to those seen in fatty acid metabolism. Given that elevations in FFA are known to influence insulin action on peripheral glucose uptake and hepatic glucose production (18), the relationships we observed are perhaps not surprising. Weight loss has also been linked with lower FFA concentrations and greater glucose disposal rates in people with type 2 diabetes (19). We observed that although improvements in both glucose and fatty acid metabolism were clearly associated with reductions in BMI, the improvements were not statistically related to changes in visceral or deep sc fat.
Given that our data do not support a detectable, independent metabolic action of rimonabant yet a number of reports seem to support an effect of peripheral CB1R antagonism in vitro and in vivo, it is useful to review possible explanations for these inconsistencies. Interest in peripheral metabolic and energy effects of CB1R antagonists arose from observations that CB1R antagonism in mice generated weight reductions not entirely explained by centrally mediated effects. These findings included continued weight loss despite only transient early reductions in food intake (20), a lack of parity in weight loss with pair-feeding controls (21–23), and weight loss in response to peripheral but not central administration (24). Furthermore, CB1R knockout mice appeared protected from weight gain despite high energy intake without increased locomotor activity (25) and had greater insulin sensitivity than wild-type littermates (22). CB1R are present in adipose tissue (25), liver (26), skeletal muscle (27), and pancreatic islets (28), providing a rational mechanism for a peripheral functional role.
Cannabinoid action in adipocytes has been related to lipoprotein lipase activity (25), mitochondrial number and function (23), oxygen consumption (4, 6), and possibly conversion of white to brown adipose tissue (29). CB1R action in skeletal muscle has been linked with altered glucose uptake (6) and adiponectin-1 receptor gene expression (30). Islet cell CB1R has been suggested to play a role in basal insulin secretion (28). Finally, improvements in fatty liver, dyslipidemia, and glucose homeostasis have been documented with peripheral CB1R antagonist action (1, 8, 31, 32). Despite these findings, the role of peripheral CB1R is not clear-cut. Similar weight loss and glucose and fatty acid metabolism changes have been observed in animals receiving CB1R antagonists and pair-fed controls (5, 25, 31, 33). Furthermore, although CB1R manipulation has been linked with adipose energy storage and expenditure (5), direct measurements of FFA, glycerol, and β-hydroxybutyrate did not differ between treatment and control animals despite weight loss differences (34). We also found modest reductions in basal metabolic rate with weight loss were not different between rimonabant and placebo treatment arms. This suggests rimonabant has no independent effect on resting energy expenditure.
It is possible that the metabolic changes observed in response to CB1R antagonism in animals are a response to anxiogenic effects. Rodents treated with CB1R antagonists have been shown to have higher anxiety scores (8) and urinary catecholamines (34). If this was associated with increase in activity, it would explain the improvements in fatty acid and glucose metabolism (35). In human studies, significant anxiogenic side effects lead to treatment withdrawal, precluding a good test of this possibility in our study. The differences observed between our study and animal data may also reflect lower clinically achievable peripheral tissue concentrations in humans. In rodents, rimonabant doses are typically 10 mg/kg · d but may be as great as 30 mg/kg · d, which are equivalent to 55–160 mg human daily doses, respectively (U.S. Food and Drug Administration drug dose conversion calculator http://www.accessdata.fda.gov/scripts/cder/onctools/animalquery.cfm. Doses above 40 mg/d were not tolerated in clinical trials, leading to a final treatment dose of 20 mg/d. Finally, it is possible that physiological differences exist between humans and these rodent models, with human peripheral CB1R playing a negligible role in the regulation of glucose and fatty acid metabolism despite potentially adequate receptor antagonism.
In this study, it was estimated that 24 complete studies per group would provide statistical power to detect a 30% improvement in insulin suppression of FFA flux in the rimonabant arm compared with placebo. After controlling statistically for the greater weight loss induced by rimonabant, we do not find evidence for an independent, peripheral effect on energy, glucose, or lipid metabolism. Our data indicate that the centrally mediated effects on satiety were the key mechanism of weight reduction and metabolic benefits in the clinical setting.
In summary, we measured insulin regulation of glucose and FFA metabolism in rimonabant- and placebo-treated patients with metabolic syndrome undergoing a 1-yr weight loss treatment program. Weight loss was associated with improvements in insulin action in both groups, yet despite significantly greater weight loss with rimonabant, the improvements were comparable between groups. This suggests any peripheral effects of CB1R antagonism with rimonabant are of minor importance compared with the central effects that induce weight loss.
Acknowledgments
This study was funded by grants from Sanofi-Aventis to Mayo Clinic, Washington University School of Medicine, University of Pittsburgh Medical Center, and the Pennington Biomedical Research Center and partially supported by National Institutes of Health Grants DK 56341 and DK50456, (Nutrition and Obesity Research Center) and UL1 RR024992 and UL1 RR024150 (Clinical and Translational Science Award).
Disclosure Summary: J.T. and M.M. have nothing to declare. S.R.S. has received grant support from Takeda; serves as a consultant to Amylin, Arena, AstraZeneca, Five Prime, NovoNordisk, Takeda, and Wyeth; and is an equity stakeholder in Jenrin Discoveries and Zafgen. S.K. is a member of the Scientific Advisory Board for Ethicon Endosurgery and Takeda Pharmaceuticals. F.T. received grant support from Sanofi-Aventis. H.A.-L. and M.J. were co-principal investigators for the VICTORIA study.
Footnotes
- Apo B
- Apolipoprotein B
- BMI
- body mass index
- BMR
- basal metabolic rate
- CB1R
- cannabinoid 1 receptor
- CT
- computed tomography
- FFA
- free fatty acid
- FFM
- fat-free mass
- HbA1c
- hemoglobin A1c
- HGO
- hepatic glucose output
- RER
- respiratory exchange ratio
- RIO
- Rimonabant in Obesity
- VICTORIA
- visceral fat reduction assessed by CT scan on rimonabant.
References
- 1. Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G. 2005. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115:1298–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrié P. 2003. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63:908–914 [DOI] [PubMed] [Google Scholar]
- 3. Pagano C, Rossato M, Vettor R. 2008. Endocannabinoids, adipose tissue and lipid metabolism. J Neuroendocrinol 20(Suppl 1):124–129 [DOI] [PubMed] [Google Scholar]
- 4. Herling AW, Kilp S, Elvert R, Haschke G, Kramer W. 2008. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed Wistar rats. Endocrinology 149:2557–2566 [DOI] [PubMed] [Google Scholar]
- 5. Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Péleraux A, Pénarier G, Soubrié P, Le Fur G, Galiègue S, Casellas P. 2005. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19:1567–1569 [DOI] [PubMed] [Google Scholar]
- 6. Liu YL, Connoley IP, Wilson CA, Stock MJ. 2005. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 29:183–187 [DOI] [PubMed] [Google Scholar]
- 7. Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Bátkai S, Marsicano G, Lutz B, Buettner C, Kunos G. 2008. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118:3160–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. 2010. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest [Erratum (2010) 120:3735] 120:2953–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pi-Sunyer FXA, Louis J., Heshmati HM, Devin J, Rosenstock J; Group R-NAS 2006. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA [Erratum (2006) 295:1252] 295:761–775 [DOI] [PubMed] [Google Scholar]
- 10. Van Gaal L, Pi-Sunyer X, Despres J, McCarthy C, Scheen A. 2008. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 31(Suppl 2):S229–S240 [DOI] [PubMed] [Google Scholar]
- 11. Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study G 2005. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134 [DOI] [PubMed] [Google Scholar]
- 12. Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, Group R-DS 2006. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet [Erratum (2006) 368:1650] 368:1660–1672 [DOI] [PubMed] [Google Scholar]
- 13. Humphreys SM, Fisher RM, Frayn KN. 1990. Micro-method for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem 27(Pt 6):597–598 [DOI] [PubMed] [Google Scholar]
- 14. Persson XM, Blachnio-Zabielska AU, Jensen MD. 2010. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 51:2761–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kury D, Keller U. 1991. Trimethylsilyl-O-methyloxime derivatives for the measurement of [6,6-2H2]-d-glucose-enriched plasma samples by gas chromatography-mass spectrometry. J Chromatogr 572:302–306 [DOI] [PubMed] [Google Scholar]
- 16. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. 2004. Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen MD, Nielsen S. 2007. Insulin dose response analysis of free fatty acid kinetics. Metabolism 56:68–76 [DOI] [PubMed] [Google Scholar]
- 18. Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. 1983. Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, Pi-Sunyer FX, Ravussin E, Group LAAR. 2010. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes 59:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. 1998. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 63:PL113–PL117 [DOI] [PubMed] [Google Scholar]
- 21. Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, Janiak P, Herbert JM. 2005. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 7:65–72 [DOI] [PubMed] [Google Scholar]
- 22. Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. 2004. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648 [DOI] [PubMed] [Google Scholar]
- 23. Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, Pasquali R, Carruba MO, Marsicano G, Lutz B, Pagotto U, Nisoli E. 2008. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 57:2028–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodríguez de Fonseca F. 2002. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22:9612–9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. 2003. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbaschek R, Garcia N, Jr, Sanyal AJ, Kunos G. 2001. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 7:827–832 [DOI] [PubMed] [Google Scholar]
- 27. Cavuoto P, McAinch AJ, Hatzinikolas G, Janovská A, Game P, Wittert GA. 2007. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem Biophys Res Commun 364:105–110 [DOI] [PubMed] [Google Scholar]
- 28. Juan-Picó P, Fuentes E, Bermúdez-Silva FJ, Javier Díaz-Molina F, Ripoll C, Rodríguez de Fonseca F, Nadal A. 2006. Cannabinoid receptors regulate Ca2+ signals and insulin secretion in pancreatic β-cell. Cell Calcium 39:155–162 [DOI] [PubMed] [Google Scholar]
- 29. Perwitz N, Wenzel J, Wagner I, Büning J, Drenckhan M, Zarse K, Ristow M, Lilienthal W, Lehnert H, Klein J. 2010. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab 12:158–166 [DOI] [PubMed] [Google Scholar]
- 30. Crespillo A, Suárez J, Bermúdez-Silva FJ, Rivera P, Vida M, Alonso M, Palomino A, Lucena MA, Serrano A, Pérez-Martín M, Macias M, Fernández-Llébrez P, Rodríguez de Fonseca F. 2011. Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J 433:175–185 [DOI] [PubMed] [Google Scholar]
- 31. Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschöp J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschöp MH. 2008. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57:2977–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, Choi SH, Yang EK, Park KJ, Chae HW, Moon HS, Kim SH, Shin YG, Yoon SH. 2010. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond) 34:547–556 [DOI] [PubMed] [Google Scholar]
- 33. Cota D, Sandoval DA, Olivieri M, Prodi E, D'Alessio DA, Woods SC, Seeley RJ, Obici S. 2009. Food intake-independent effects of CB1 antagonism on glucose and lipid metabolism. Obesity 17:1641–1645 [DOI] [PubMed] [Google Scholar]
- 34. Mølhøj S, Hansen HS, Schweiger M, Zimmermann R, Johansen T, Malmlöf K. 2010. Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur J Pharmacol 646:38–45 [DOI] [PubMed] [Google Scholar]
- 35. Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. 1993. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 265:E380–E391 [DOI] [PubMed] [Google Scholar]