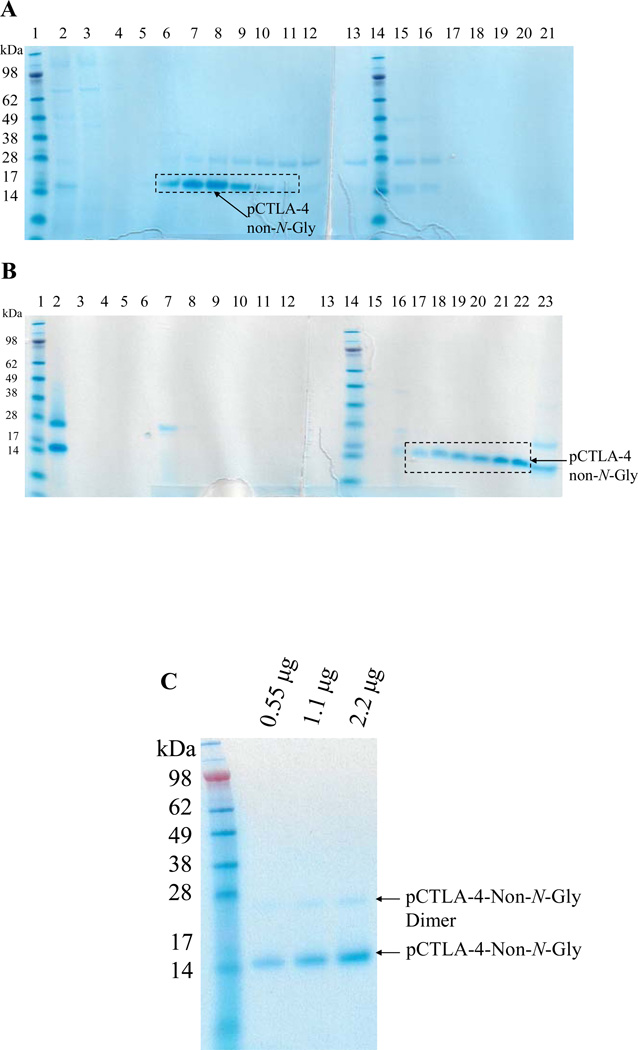
Figure 4.
Purification for non-N-glycosylated soluble porcine CTLA-4: A) First step purification using Ni-Sepharose 6 fast flow resin. Lane 1 and 14: protein markers; Lane 2: sample; Lane 3: flowthrough; Lane 4: washing; Lane 5–12: eight 50 mL elution fractions using 100 mM imidazole; Lane 13 and 15–21: eight 50 mL elution fractions using 200 mM imidazole. B) Second step purification using strong anion-exchange resin Poros 50HQ. Lane 1 and 14: protein markers; Lane 2: sample; Lane 3: flowthrough; Lane 4–5: two 40 mL washing fractions; Lane 6–13: eight 10 mL elution fractions using 100 mM sodium borate; Lane 15–22: eight 10 mL elution fractions using 200 mM sodium borate. Lane 23: flushing using 1 M NaCl. C) 12% NuPAGE SDS gel analysis for the final product of non-N-glycosylated soluble porcine CTLA-4 (14.1 kDa). The weak band seen at approximately 28 kDa is a covalent homodimer of the non-N-glycosylated soluble porcine CTLA-4 generated by disulfide bond formation (analyzed under reduced conditions using DTT and beta-mercaptoethanol, data not shown).