Abstract
Objective
To determine whether higher body mass index (BMI) is associated with more adverse lower extremity muscle characteristics at baseline and more adverse changes in muscle over time among participants with lower extremity peripheral arterial disease (PAD).
Design
Longitudinal, observational study.
Setting
Academic medical center in Chicago.
Subjects
Participants were 425 men and women with PAD and 261 without PAD.
Interventions
Computed Tomography was used to measure calf muscle characteristics at baseline and every two years. Knee extension isometric strength, power, and six-minute walk were measured at baseline and annually. Baseline BMI categories were ideal (20-25 kg/m2), overweight (>25-30 kg/m2), and obese (>30 kg/m2). Analyses adjust for age, race, gender, ankle brachial index (ABI), comorbidities, and other covariates.
Results
At baseline, among participants with PAD, higher BMI was associated with greater calf muscle area (ideal BMI: 5181 mm2, overweight: 5513 mm2, obese: 5695 mm2, p trend=0.0009), higher calf muscle percent fat (6.38%, 10.28%, 17.44% respectively, p trend<0.0001), lower calf muscle density (p trend<0.0001), and higher isometric knee extension strength (p trend=0.015). Among participants with PAD, higher BMI was associated with greater declines in calf muscle area p trend=0.030) and greater increases in calf muscle percent fat (p trend=0.023). Among participants without PAD, there were no significant associations of baseline BMI with changes in lower extremity muscle outcomes over time.
Conclusions
Among PAD participants, higher BMI is associated with greater calf muscle area at baseline. However, higher BMI is associated with more adverse calf muscle density and percent fat at baseline and greater declines in calf muscle area over time.
INTRODUCTION
Peripheral arterial disease (PAD) is a common, debilitating condition.1 PAD is associated with functional limitation and disability2,3 for which there are few effective treatments.
Prior research demonstrates that higher body mass index (BMI) is associated with greater functional decline in people with PAD.4 The pathophysiologic basis of this association is unclear. However, prior study demonstrates that adverse calf muscle characteristics are associated with greater functional impairment and faster functional decline in men and women with PAD.5,6 The previously demonstrated associations of higher BMI with greater functional decline in PAD may in part be mediated by obesity-related changes in calf muscle composition. However, associations of higher BMI with calf muscle characteristics have not been reported previously in men and women with PAD.
We studied associations of baseline BMI with calf muscle area, calf muscle density, calf muscle percent fat, knee extension strength, and knee extension power among participants with PAD. We hypothesized that higher BMI would be associated with more adverse lower extremity muscle characteristics at baseline and more adverse changes in muscle characteristics over time. To determine whether associations identified were unique to individuals with PAD, we also studied associations of BMI with baseline muscle characteristics and changes in these characteristics over time among individuals without PAD. Finally, among participants with PAD, we used statistical modeling to determine whether associations of higher baseline BMI with more rapid functional decline in PAD may be mediated by associations of baseline BMI with adverse calf muscle characteristics, knee extension isometric strength, and knee extension power.
METHODS
Patient Identification
The protocol was approved by the Institutional Review Boards at Northwestern University Feinberg School of Medicine and Catholic Health Partners Hospital. Participants were men and women with and without PAD participating in the Walking and Leg Circulation Study II (WALCS-II).5 PAD participants were consecutively identified from three non-invasive vascular laboratories in the Chicago area.5 A small number of PAD participants were identified from among patients in a general medical practice found to have a low ABI, consistent with PAD.5 Participants without PAD were identified from among consecutive patients with normal lower extremity arterial tests in the vascular laboratory and from among consecutive patients in the general internal medicine practice who were found to have a normal ABI.5 Data were collected between 11/06/2002 and 10/16/2009. All participants gave written informed consent. Participants completed a baseline visit and up to four annual follow-up visits. Calf muscle characteristics were measured by Computed Tomography (CT) at baseline and at two and four-year follow-up. Isometric knee extension strength and knee extension power were measured at baseline and at one, two and three-year follow-up.
Inclusion and Exclusion Criteria
Participants were age 59 or older at baseline. PAD was defined as ABI <0.90.5 Absence of PAD was defined as an ABI of 0.90 to 1.30.5 Exclusion criteria for WALCS II have been described.5 Potential participants with ABI ≥ 1.30 were excluded, since these individuals could have stiffened lower extremity vessels. Potential participants with ABI 0.90 to 1.30 and history of lower extremity revascularization were excluded. Subjects with life expectancy <12 months were excluded to allow for adequate follow-up. Those with dementia and those with a Mini-Mental Status Examination score <23 were excluded.7 Participants who were nursing home residents, wheelchair-bound, or who had foot or leg amputations were excluded because of severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Potential participants with BMI <20 kg/m2 were excluded because of the small number of potential participants who met this criterion and because BMI <20 kg/m2 is typically classified distinctly from BMI 20-25 kg/m2.
Body Mass Index
Height and weight were measured at baseline. Weight was re-measured at follow-up visits. BMI was calculated as weight in kg/height in meters2. BMI categories were defined as ideal (BMI 20-25 kg/m2), overweight (BMI >25-30 kg/m2) and obese (BMI >30 kg/m2).
Ankle Brachial Index
After participants rested supine for five minutes, systolic pressures were measured using a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) in the brachial, dorsalis pedis and posterior tibial arteries of the right and left extremities. All pressures were measured twice. The ABI was calculated for each leg by dividing the average of the dorsalis pedis and posterior tibial artery pressures in each leg by the average of the four (i.e. left and right) brachial pressures.5 When one brachial pressure was higher than the opposite arm for both measurement sets, and the two brachial pressures differed by >10 mmHg in at least one measurement set, the arm with highest pressure was used in the denominator for calculating the ABI for the left and right legs, since subclavian stenosis was potentially present in these instances.8 The same denominator was used for calculating the ABI in the left and right legs. Lowest leg ABI was included in analysis. Calf muscle characteristics were evaluated in the leg with lowest baseline ABI.
Calf Muscle Characteristics
Calf muscle characteristics were obtained using a CT scanner (LightSpeed, General Electric Medical Systems, Waukesha, WI, USA). Cross-sectional images of the calves were obtained at 66.7% of the distance from the distal to the proximal tibia.5 The muscle outline was traced manually on the cross-sectional CT images, excluding subcutaneous fat and bone, using BonAlyse software.5,6 BonAlyse software quantifies muscle area by summing voxels corresponding to muscle (9 to 271 mg/cm3) and excluding voxels corresponding to fat (-270 to 8 mg/cm3).5,6 Estimates of muscle area using this method are highly correlated with direct anatomic measurements in previous cadaveric studies.9 Intra-muscular fat was quantified by summing voxels corresponding to fat within the demarcated muscle compartment. Muscle density is a measure of muscle quality, and was calculated as the mean number of voxels within the range corresponding to muscle (9 to 271 mg/cm3) per volume.
Isometric Knee Extension Strength
Isometric knee extension strength was recorded using a computer-linked strength chair fitted with leg attachments and transducers to measure isometric knee extension over five seconds (Good Strength Chair, Metitur Oy, Jyvasklya, Finland).10 Participants were instructed to build to their maximum strength over two seconds and maintain maximum strength for the final three seconds of the test. Maximum strength from two trials was used in analyses.10
Knee extension Power
Knee extension power was obtained by asking subjects to push a footplate connected to a flywheel using maximal effort.11 Final flywheel velocity was recorded by an optoswitch attached to a microcomputer, and was used to calculate knee extension power in Watts.11 Five to nine trials were performed for each subject. Testing was stopped when the two highest power measurements were within 5% of each other or when the participant had completed nine tests. 11
Functional outcomes
Functional outcomes were measured using a standardized protocol at baseline and at each annual follow-up visit. The six minute walk was performed using standard methods.2,3,5,6 The participant walked back and forth along a 100 foot hallway, after instructions to cover as much distance as possible.2,3 Four meter walking velocity was recorded as walking velocity over 4 meters at “usual” and “fastest” walking speeds, respectively. The faster of two walks at each pace was included in analyses.2,3,5,6,12 Repeat chair rise was the time required for five consecutive rises from a seated position in a straight-backed chair with arms folded across the chest.12 Standing balance was recorded as ability to perform three increasingly difficult standing positions for ten seconds each as previously described.12
The Short Physical Performance Battery (SPPB) (score range from 0-12) was recorded as the sum total of three separate scores (ranging from 0-4), assigned for performance in repeated chair rises, standing balance and usual paced four meter walking velocity. A score of zero was assigned for any task a participant was unable to complete. Remaining scores for each task were based upon quartiles of performance from more than 6,000 participants in the Established Populations for the Epidemiologic Study for the Elderly.12
Comorbidities
Comorbidities assessed were hypertension, diabetes mellitus, angina, myocardial infarction, stroke, heart failure, pulmonary disease, cancer, spinal stenosis, disk disease and knee and hip osteoarthritis. Most comorbidities were documented using algorithms developed for the Women's Health and Aging Study and the Cardiovascular Health Study.13 These comorbidity algorithms combined data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire. History of hypertension was based on a physician's indication of hypertension on the primary care physician questionnaire or patient report of physician-diagnosed hypertension. American College of Rheumatology criteria were used to identify knee and hip osteoarthritis.14,15
Other Measures
Cigarette smoking history was based on patient report. Activity level was based on patient report of number of blocks walked. Occurrence of lower extremity revascularization, knee replacement surgery, or hip replacement surgery were measured during follow-up based on patient-report, medical record review, and a primary care physician questionnaire. If any of these sources reported hip or knee replacement surgery, the participant was considered to have had the procedure. Patient report of lower extremity revascularization required confirmation with medical record review or a primary care physician questionnaire.
Statistical Analyses
Baseline characteristics of participants with and without PAD across the defined BMI categories were compared using chi-square tests for categorical variables and general linear models for continuous variables. Among participants with and without PAD, respectively, analyses of covariance and statistical tests for trend were used to compare each baseline calf muscle characteristic, baseline knee extension strength, and baseline knee extension power across BMI categories, adjusting for age, race, sex, ABI (PAD participants only), smoking, physical activity, and comorbidities. We adjusted for tibia length in analyses of calf muscle area and knee extension measures.16
Associations of baseline BMI categories with average annual change in functional performance, calf muscle characteristics, knee extension strength, and knee extension power were analyzed using mixed-effects models, in which a subject-specific random effect was used to account for the potential correlations among successive annual differences in each outcome measure. Dependent variables in each mixed-effect regression analysis were the successive annual changes in functional performance and muscle outcomes, respectively. Analyses adjusted for age, race, gender, ABI (PAD participants only), smoking, comorbidities, activity level, tibia length (muscle area and knee extension measures only), lower extremity revascularization during follow-up, hip or knee replacement during follow-up, and prior year functional performance (for functional outcomes) or prior year muscle measure (for muscle outcomes).
Among participants with PAD, adjusted analyses of associations of baseline BMI with average annual functional decline were repeated with additional adjustments for baseline calf muscle percent fat, baseline calf muscle density, changes in calf muscle area, change in calf muscle percent fat, and all of these calf muscle measures simultaneously. These additional analyses were performed to determine whether associations of BMI with decline in functional performance were mediated by these muscle characteristics. In longitudinal analyses, BMI was a time-dependent variable, in which the BMI value used in analyses was updated for each follow-up visit. Statistical analyses were performed using SAS statistical software (version 9.2, SAS Institute Inc, Cary, NC).
RESULTS
Of 729 WALCS-II participants who completed baseline testing, 463 were PAD participants and 279 were non-PAD participants. Of these, 425 PAD subjects and 261 non-PAD subjects met inclusion criteria and were included in baseline analyses (Figure 1). Of these, 357 with PAD and 229 without PAD were eligible for longitudinal analyses (Figure 1). Mean follow-up was 3.94 years.
Figure 1.
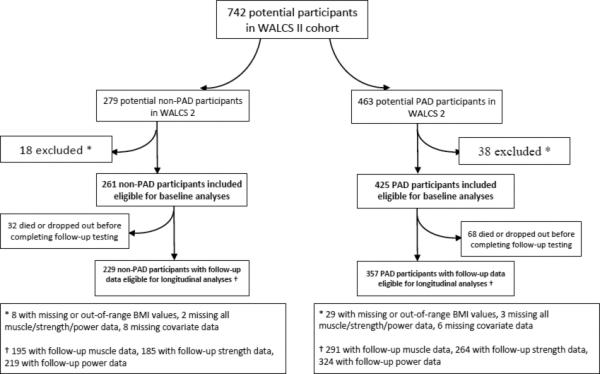
Participants with and without Peripheral Arterial Disease in the Walking and Leg Circulation Study II
Among the 425 PAD subjects who completed baseline testing, the average age was 74.9 ± 8.2 years, mean ABI was 0.63 ± 0.15, and 104 (24%) had classic symptoms of intermittent claudication. Among the 264 participants without PAD who completed baseline testing, average age was 71.5 years ± 7.5 and mean ABI was 1.09±0.09. Among participants with PAD, 57.2% were taking cholesterol-lowering medication and six percent were taking cilostazol at baseline. Among participants without PAD, 30.7% were taking cholesterol-lowering medication.
Table I shows patient characteristics according to baseline BMI among participants with and without PAD. Among participants with and without PAD, higher BMI was associated with a higher prevalence of diabetes mellitus, greater calf muscle area, higher calf muscle percent fat and lower calf muscle density. Among participants with PAD, higher BMI was also associated with younger age, higher isometric knee extension strength and higher knee extension power. Among participants without PAD, higher BMI values were associated with African-American race, lower ABI values, and a higher prevalence of knee osteoarthritis (Table 1).
Table 1.
Baseline characteristics of participants with and without peripheral arterial disease according to baseline body mass index (n=686)
| Participants with Peripheral Arterial Disease | Participants without Peripheral Arterial Disease | |||||||
|---|---|---|---|---|---|---|---|---|
| BMI 20-25 kg/M2 (n=108) | BMI 25-30 Kg/M2 (n=184) | BMI >30 Kg/M2 (n=134) | P trend | BMI 20-25 kg/M2 (n=61) | BMI 25-30 Kg/M2 (n=98) | BMI >30 Kg/M2 (n=105) | P trend | |
| Age, years (SD) | 76.4 (8.9) | 75.2 (8.6) | 73.3 (6.7) | 0.003 | 72.9 (8.2) | 71.5 (6.8) | 70.6 (7.6) | 0.056 |
| Black race, % | 17.6 | 11.4 | 22.6 | 0.232 | 8.2 | 23.2 | 23.8 | 0.027 |
| Male sex, % | 43.5 | 63.0 | 54.5 | 0.133 | 34.4 | 50.0 | 43.8 | 0.355 |
| Ankle Brachial Index (SD) | 0.61 (0.15) | 0.64 (0.15) | 0.63 (0.16) | 0.202 | 1.12 (0.09) | 1.10 (0.09) | 1.06 (0.10) | <0.001 |
| Diabetes mellitus, % | 23.2 | 30.4 | 46.3 | <0.001 | 13.1 | 15.8 | 36.2 | <0.001 |
| Angina, % | 30.2 | 39.0 | 36.1 | 0.368 | 15.00 | 17.89 | 24.27 | 0.134 |
| Myocardial Infarction (%) | 25.0 | 22.9 | 32.3 | 0.169 | 14.8 | 11.2 | 18.1 | 0.446 |
| Stroke (%) | 25.9 | 19.6 | 22.6 | 0.583 | 9.84 | 9.47 | 6.67 | 0.440 |
| Heart Failure (%) | 25.9 | 28.3 | 34.3 | 0.135 | 11.5 | 13.7 | 16.2 | 0.393 |
| Knee osteoarthritis (%) | 13.0 | 9.2 | 17.3 | 0.260 | 14.8 | 13.7 | 26.7 | 0.035 |
| Hip osteoarthritis (%) | 4.6 | 0.00 | 7.5 | 0.156 | 0.00 | 4.2 | 5.7 | 0.089 |
| Calf muscle area, mm2 (SD) | 4,928 (901) | 5,649 (1,388) | 5,742 (1,687) | <.0001 | 5,350 (1,168) | 5,806 (1,530) | 6,603 (1,567) | <.0001 |
| Calf muscle density, gm/cm3 (SD) | 33.4 (3.6) | 33.2 (3.8) | 30.9 (4.5) | <.0001 | 34.9 (3.8) | 34.1 (3.6) | 32.5 (4.1) | <.0001 |
| Calf muscle percent fat, % (SD) | 7.1 (7.0) | 9.9 (10.6) | 17.3 (17.5) | <.0001 | 6.9 (9.9) | 9.5 (12.0) | 12.2 (12.8) | 0.005 |
| Isometric knee extension strength, newtons (SD) | 237 (85) | 286 (112) | 297 (118) | <0.001 | 268 (97) | 298 (133) | 298 (109) | 0.177 |
| Knee extension power, watts (SD) | 81 (41) | 95 (50) | 103 (60) | 0.002 | 104 (44) | 128 (70) | 115 (58) | 0.481 |
SD = Standard Deviation
Figure 2 shows associations of baseline BMI with baseline calf muscle characteristics and knee extension strength among participants with PAD, adjusting for age, race, gender, ABI, smoking, comorbidities, physical activity, and tibia length (muscle area and knee extension measures only). Higher baseline BMI was associated significantly with higher calf muscle area and higher isometric knee extension strength. Higher baseline BMI was also associated with higher calf muscle percent fat and lower calf muscle density.
Figure 2. Adjusted associations of baseline body mass index with baseline calf muscle characteristics among men and women with PAD (n=425)**.
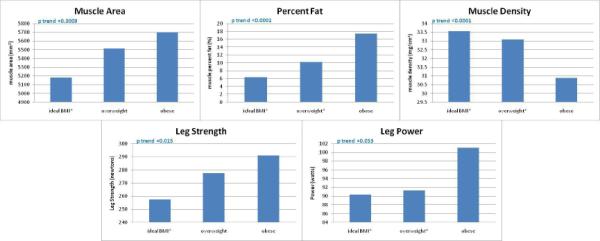
**Data are adjusted for age, race, gender, ABI, smoking, comorbidities, activity level and tibia length (muscle area and knee extension measures only). BMI categories were defined as ideal (BMI 20-25 kg/m2), overweight (BMI >25-30 kg/m2) and obese (BMI >30 kg/m2). * significant pair-wise comparison with obese BMI category. Pair-wise p-values were <0.001 (percent fat, muscle density), 0.0009 (muscle area), 0.014 (isometric knee extension strength), 0.048 (knee extension power).
N=412 for muscle area, percent fat, muscle density analyses. N=313 for leg strength analysis.
N=384 for leg power analysis.
Figure 3 shows associations of BMI with average annual changes in lower extremity muscle characteristics, adjusting for age, race, gender, ABI, smoking, comorbidities, activity level, lower extremity revascularization during follow-up, hip or knee replacement surgery during follow-up, tibia length (muscle area and knee extension measures only) and prior year muscle measurements. Higher baseline BMI was associated with greater decline in calf muscle area (p trend =0.030) and greater increase in calf muscle percent fat (p trend=0.023). There were no associations between higher baseline BMI and change in calf muscle density or leg strength measures (Figure 3).
Figure 3. Adjusted associations of body mass index with change in calf muscle characteristics among participants with peripheral arterial disease.
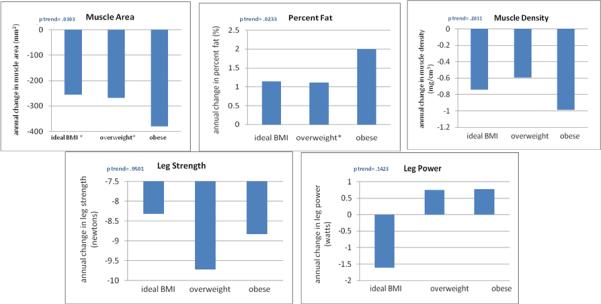
** Data are adjusted for age, race, gender, ABI, smoking, comorbidities, activity level, tibia length (muscle area and leg strength measures only), lower extremity revascularization during follow-up, hip or knee replacement during follow-up, and prior calf muscle characteristics. BMI categories were defined as ideal (BMI 20-25 kg/m2), overweight (BMI >25-30 kg/m2) and obese (BMI >30 kg/m2). * denotes a significantly different value as compared to the obese BMI category. For muscle area, the pair-wise p-value for the comparison between ideal BMI and obese is 0.043. The pairwise p value for the comparison between overweight BMI and obese is .0254. For calf muscle percent fat, the pairwise p value for the comparison between obese and overweight is 0.0261.
N=291 for muscle area, percent fat, muscle density analyses. N=264 for leg strength analysis.
N=324 for leg power analysis.
Table II shows associations of baseline BMI with average annual functional decline, adjusting for age, race, gender, ABI, smoking, comorbidities, activity level, lower extremity revascularization during follow-up, hip or knee replacement during follow-up, and prior year performance. Model 1a in Table II shows associations of baseline BMI with average annual functional decline, among participants with baseline calf muscle characteristic data. Higher baseline BMI was associated with greater declines in the SPPB (p trend= 0.025), usual-paced walking velocity (p trend= 0.001), fastest-paced walking velocity (p trend<0.001), and six-minute walk (p trend=0.002) (Model 1a, Table II). The significant association between higher BMI and greater decline in the SPPB was attenuated and no longer statistically significant after additional adjustment for baseline calf muscle percent fat and baseline calf muscle density (Table II). Associations of higher baseline BMI with faster declines in walking velocity and the six-minute walk were somewhat attenuated but remained highly statistically significant after additional adjustment for baseline calf muscle percent fat and baseline calf muscle density, respectively (Table II). These findings suggest that more adverse calf muscle percent fat and calf muscle density at baseline among more obese PAD participants may contribute to associations of higher baseline BMI with faster decline in the SPPB, but may not contribute substantially to associations of higher baseline BMI with faster decline in walking velocity or the six-minute walk. Model 1a was repeated among participants with follow-up data on changes in calf muscle characteristics over time (Table II). When the sample size was limited to patients with follow-up calf muscle data, higher BMI was associated significantly with greater declines in the SPPB and the two measures of walking velocity, but not with the six-minute walk (Table II, Models 1b). Associations of higher BMI values with greater declines in the SPPB and walking velocity remained statistically significant and were attenuated only to a small degree after adjusting for change in percent fat and change in calf muscle area. Associations of BMI with decline in the SPPB and usual-paced walking velocity were attenuated and no longer statistically significant after additional adjustment for baseline calf muscle measures and changes in calf muscle measures (Table II). However, the association of BMI with decline in fast-paced four-meter walking velocity remained statistically significant after adjusting for both baseline calf muscle measures and changes in calf muscle measures (Table II). Associations of BMI with decline in six-minute walk became statistically significant after additional adjustment for calf muscle characteristics (Table II). These findings suggest that associations of higher baseline BMI with more adverse calf muscle characteristics at baseline and changes in calf muscle over time may contribute to faster rates of decline in the SPPB among more obese PAD participants.
Table 2.
Baseline Body Mass Index and Functional Decline among Participants with Peripheral Arterial Disease
| Associations of Body Mass Index with Functional Decline with and without additional adjustment for baseline calf muscle characteristics | ||||
|---|---|---|---|---|
| BMI 20-25 | BMI 25-30 | BMI >30 | P trend | |
| Short Physical Performance Battery Decline (n=304) | n=76 | n=138 | n=90 | |
| Model 1a | -0.32[-0.53, -0.11] | -0.35 [-0.53, -0.17] | -0.57 [-0.78, -0.37] | 0.025 |
| Model 1a plus adjustment for baseline percent fat | -0.35 [-0.57, -0.136] | -0.35 [-0.54, -0.17] | -0.52[-0.732, -0.32] | 0.159 |
| Model 1a plus adjustment for baseline calf density | -0.33 [-0.55, -0.117] | -0.36 [-0.54, -0.17] | -0.55 [-0.753, -0.34] | 0.064 |
| Usual Walking Velocity Decline, m/s (n=337) | n=81 | n=152 | n=104 | |
| Model 1a | -0.03[-0.04, -0.01] | -0.04 [-0.05, -0.02] | -0.05 [-0.06, -0.04] | 0.001 |
| Model 1a plus adjustment for baseline percent fat | -0.03[-0.04, -0.02] | -0.04 [-0.05, -0.02] | -0.05 [-0.06, -0.03] | 0.004 |
| Model 1a plus adjustment for baseline calf density | -0.03 [-0.04, -0.01] | -0.04 [-0.05, -0.02] | -0.05 [-0.06, -0.04] | 0.002 |
| Fastest Walking Velocity Decline, m/s (n=332) | n=81 | n=149 | n=102 | |
| Model 1a | -0.02 [-004, -0.01] | -0.03 [-0.05, -0.01] | -0.05 [-0.06, -0.03] | <0.001 |
| Model 1a plus adjustment for baseline percent fat | -0.02 [-0.04, -0.01] | -0.03 [-0.05, -0.01] | -0.05 [-0.06, -0.03] | 0.001 |
| Model 1a plus adjustment for baseline calf density | -0.02 [-0.04, -0.01] | -0.03 [-0.05, -0.02] | -0.05 [-0.06, -0.03] | 0.001 |
| Six Minute Walk Decline, ft (n=323) | n=80 | n=146 | n=97 | |
| Model 1a | -32.2 [-57.4, -7.1] | -45.7 [-66.1, -25.3] | -76.3 [-101.0, -51.7] | 0.002 |
| Model 1a plus adjustment for baseline percent fat | -33.2 [-58.7, -7.8] | -45.6 [-66.13, -25.1] | -74.9 [-100.0, -49.8] | 0.004 |
| Model 1a plus adjustment for baseline calf density | -32.3 [-57.6, -7.0] | -45.7 [-66.22, -25.2] | -76.2 [-101.3, -51.1] | 0.002 |
| Associations of Body Mass Index with Functional Decline with and without additional adjustment for changes in calf muscle characteristics over time | ||||
|---|---|---|---|---|
| BMI 20-25 | BMI 25-30 | BMI >30 | P trend | |
| Short Physical Performance Battery Decline (n=234) | n=57 | n=109 | n=68 | |
| Model 1b | -0.74 [-1.3, -0.24] | -0.86 [-1.4, -0.38] | -1.1 [-1.6, -0.62] | 0.003 |
| Model 1b plus adjustment for change in percent fat | -0.75 [-1.3, -0.26] | -0.86 [-1.3, -0.38] | -1.1 [-1.6, -0.62] | 0.006 |
| Model 1b plus adjustment for change in calf area | -0.77 [-1.3, -0.28] | -0.86 [-1.3, -0.39] | -1.1 [-1.5, -0.60] | 0.012 |
| Model 1b plus baseline muscle density, baseline percent fat, change in percent fat, change in muscle area | -0.82 [-1.3, -0.32] | -0.89 [-1.4, -0.41] | -1.1 [-1.5, -0.58] | 0.081 |
| Usual Walking Velocity Decline, m/s (n=257) | n=60 | n=120 | n=77 | |
| Model 1b | -0.02 [-0.05, 0.02] | -0.02 [-0.06, 0.01] | -0.04 [-0.07, -0.00] | 0.017 |
| Model 1b plus adjustment for change in percent fat | -0.02 [-0.05, 0.02] | -0.02 [-0.06, 0.01] | -0.04 [-0.07, -0.00] | 0.019 |
| Model 1b plus adjustment for change in calf area | -0.02 [-0.05, 0.02] | -0.02 [-0.05, 0.01] | -0.04 [-0.07, -0.00] | 0.023 |
| Model 1b plus baseline muscle density, baseline percent fat, change in percent fat, change in muscle area | -0.02 [-0.05, 0.02] | -0.02 [-0.05, 0.01] | -0.03 [-0.07, 0.00] | 0.061 |
| Fastest Walking Velocity Decline, m/s (n=256) | n=60 | n=119 | n=77 | |
| Model 1b | -0.03 [-0.07, 0.01] | -0.05 [-0.08, -0.01] | -0.05 [-0.09, -0.01] | 0.009 |
| Model 1b plus adjustment for change in percent fat | -0.03 [-0.07, 0.01] | -0.04 [-0.08, -0.01] | -0.05 [-0.09, -0.01] | 0.010 |
| Model 1b plus adjustment for change in calf area | -0.03 [-0.07, 0.01] | -0.04 [-0.08, -0.01] | -0.05 [-0.09, -0.01] | 0.010 |
| Model 1b plus baseline muscle density, baseline percent fat, change in percent fat, change in muscle area | -0.03 [-0.07, 0.01] | -0.04 [-0.08, -0.00] | -0.05 [-0.09, -0.01] | 0.013 |
| Six Minute Walk Decline, ft (n=252) | n=60 | n=116 | n=76 | |
| Model 1b | -23.5 [-66.8, 19.9] | -37.8 [-78.1, 2.6] | -64.7 [-107.3, -22.1] | 0.109 |
| Model 1b plus adjustment for change in percent fat | -21.8 [-64.8, 21.3] | -34.9 [-75.1, 5.3] | -61.4 [-103.8, -18.9] | 0.013 |
| Model 1b plus adjustment for change in calf area | -21.5 [-64.7, 21.7] | -35.5 [-75.8, 4.8] | -61.3 [-103.9, -18.6] | 0.012 |
| Model 1b plus baseline muscle density, baseline percent fat, change in percent fat, change in muscle area | -24.1 [-67.3, 19.2] | -35.1 [-75.2, 5.0] | -60.4 [-103.0, -17.5] | 0.037 |
Data shown are average annual decline in functional performance and 95% confidence intervals across baseline BMI categories. Model 1 adjusts for age, race, gender, ABI, smoking, comorbidities, physical activity, lower extremity revascularization during follow-up, hip or knee replacement during follow-up, and prior year performance. Model 1a is limited to all participants with baseline muscle data. Model 1b is limited to participants with follow-up muscle data.
Among participants without PAD, higher BMI was associated with higher calf muscle area and lower calf muscle density at baseline, adjusting for age, race, sex, smoking, physical activity, comorbidities and tibia length (calf muscle area and knee extension outcome only) (Table 3). Among participants without PAD, higher BMI was not associated with calf muscle percent fat or lower extremity strength measures after adjusting for confounders (Table 3). Among participants without PAD, there were no associations of baseline BMI with change in lower extremity muscle measures over time, adjusting for age, sex, race, smoking, physical activity, comorbidities, prior year muscle measures, and hip or knee replacement during follow-up (Table 3).
Table 3.
Associations of Body Mass Index with Baseline Lower Extremity Muscle Characteristics and change in Lower Extremity Muscle Characteristics among Participants without PAD
| BMI 20-25 Kg/M2 | BMI 25-30 Kg/M2 | BMI >30 Kg/M2 | P trend | |
|---|---|---|---|---|
| Baseline Data | ||||
| Muscle area (millimeters2) | N=60 5401 (5,070- 5,732) |
N=92 5731 (5,474- 5,988) |
N=104 6636 (6,387- 6,885) |
<0.001 |
| Percent fat | N=60 8.42 (5.33 - 11.50) |
N=93 9.34(6.96 - 11.73) |
N=104 11.46 (9.14 -13.78) |
0.117 |
| Muscle Density | N=60 34.5 (33.5 - 35.4) |
N=93 34.1(33.4 - 34.9) |
N=104 32.8(32.1-33.5) |
0.005 |
| Isometric Knee Extension Strength | N=51 277 (252 - 301) |
N=78 293 (274 - 312) |
N=83 297 (278-316) |
0.226 |
| Leg Power | N=56 107 (94 - 120) |
N=85 125 (115 - 135) |
N=99 117(107-126) |
0.404 |
| Longitudinal Data (i.e. average annual change in each leg muscle measure) | ||||
| Muscle area (millimeters2) | n=45 -239[-320, -158] |
n=69 -229 [-291, -168] |
n=80 -289 [-350, -228] |
0.214 |
| Percent fat | n=45 1.14 [0.47, 1.81] |
n=70 1.09 [0.58, 1.59] |
n=80 1.48 [1.00, 1.97] |
0.112 |
| Muscle Density | n=45 -0.70 [-1.09, -0.31] |
n=70 -0.32 [-0.61, -0.03] |
n=80 -0.65 [-0.94, -0.37] |
0.456 |
| Isometric Knee Extension Strength | n=32 -8.60 [-16.70, -0.49] |
n=59 -7.39 [-12.98, -1.80] |
n=61 -3.96 [-10.46, 2.54] |
0.875 |
| Leg Power | n=49 -4.68 [-9.09, -0.27] |
n=73 -1.25 [-4.60, 2.11] |
n=89 -0.26 [-3.54, -3.03] |
0.388 |
Baseline associations are adjusted for age, sex, race, smoking, physical activity, comorbitities (calf muscle area outcome only). Longitudinal associations are adjusted for age, sex, race, smoking, physical activity, comorbidities, prior year muscle measurement, and hip or knee replacement during follow-up.
DISCUSSION
Results reported here demonstrate that higher baseline BMI was associated with higher baseline calf muscle percent fat and lower baseline calf muscle density among men and women with PAD. However, higher baseline BMI was also associated with higher calf muscle area and better knee extension isometric strength. In longitudinal analyses, higher BMI at baseline was associated with greater decline in calf muscle area and greater increases in calf muscle percent fat, but no significant changes in calf muscle density, knee extension isometric strength or knee extension power compared to lower baseline BMI values. Among participants without PAD, higher BMI was associated with greater calf muscle area and lower calf muscle density at baseline. There were no associations of baseline BMI with change in lower extremity muscle characteristics over time among participants without PAD.
The association of higher BMI with lower baseline calf muscle density at baseline among participants with and without PAD and the association of higher BMI with greater baseline calf muscle percent fat at baseline among individuals with PAD may reflect poorer muscle quality in obese participants with and without PAD, despite higher muscle quantity, measured by greater calf muscle area. The associations of higher BMI with higher calf muscle area among participants with and without PAD and the association of higher BMI with better knee extension strength among participants with PAD at baseline may reflect the need for greater muscle quantity or strength to support higher body weight in obese individuals. Higher muscle area may compensate for lower muscle density (i.e. poorer muscle quality) among obese participants.
Among participants with PAD, higher baseline BMI was associated with greater declines in SPPB score, usual and fast-paced walking velocity, and six-minute walk performance after adjusting for confounders. The association of higher BMI with faster decline in the SPPB was attenuated after additional adjustment for the adverse calf muscle characteristics associated with higher BMI values. This finding suggests that adverse associations of BMI with baseline calf muscle characteristics and changes in these characteristics over time may partially explain associations of higher BMI with greater declines in the SPPB. However, among participants with PAD, associations of higher BMI with faster declines in walking velocity and the six-minute walk test were not substantially attenuated by additional adjustments for baseline adverse muscle characteristics or changes in muscle characteristics associated with higher BMI values. Thus, these associations of higher BMI with faster declines in walking velocity and six-minute walk are not likely to be explained by the associations of higher BMI with more adverse calf muscle characteristics at baseline or changes in these characteristics over time.
Our findings suggest, for the first time, that obesity is associated with lower calf muscle density, higher calf muscle percent fat and greater declines in calf muscle area as measured by CT in men and women with PAD. Our findings suggest that the combination of PAD with higher BMI values may be particularly adverse, since higher BMI values were associated with greater declines in calf muscle area and greater increases in calf muscle percent fat only among PAD participants but not among participants without PAD.
The significance of the faster decline in calf muscle area among PAD participants with higher baseline BMI is unclear, since obese participants with PAD did not experience greater decline in knee extension strength or knee extension power. However, our longitudinal measures of lower extremity strength did not include changes in plantarflexion, which is expected to better reflect declines in calf muscle area over time, since calf muscle is responsible for plantarflexion strength while muscles proximal to the calf are responsible for knee extension.
This study has limitations. First, the study is observational. Causal inferences cannot be made. Second, of the 426 eligible PAD participants who completed baseline testing, only 359 had follow-up data for these analyses. Third, although our cohort was followed prospectively for four years, longer follow-up may be needed to detect additional adverse changes in lower extremity muscle associated with obesity in PAD. Fourth, we did not have longitudinal data on plantarflexion strength. Fifth, we did not have data on insulin resistance which may be a mediator of the associations reported here. Sixth, we did not collect CT images of upper extremity muscle. Therefore, we cannot determine whether associations of baseline BMI with changes in lower extremity muscle characteristics are unique to the lower extremities.
Our findings demonstrate that higher BMI is associated with more adverse calf muscle density and percent fat at baseline and greater decline in calf muscle area at four-year follow-up among men and women with PAD. Further investigation is needed to determine whether weight loss among obese patients with PAD can protect against adverse muscle characteristics at baseline and adverse changes in calf muscle characteristics over time. Our study suggests that PAD patients should be encouraged to maintain an ideal body weight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American College of Cardiology Annual Meeting, March 14-16 2010 in Atlanta, Georgia
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study). Journal of the American College of Cardiology. 2009;53:1056–62. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Tian L, Ferrucci L, Liu K, Guralnik JM, Liao Y, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. Journal of the American Geriatrics Society. 2008;56:724–9. doi: 10.1111/j.1532-5415.2008.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Criqui MH, Ferrucci L, Guralnik JM, Tian L, Liu K, et al. Obesity, weight change, and functional decline in peripheral arterial disease. Journal of Vascular Surgery. 2006;43:1198–204. doi: 10.1016/j.jvs.2006.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. Journal of the American Geriatrics Society. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, Liao Y, Criqui MH. Pathophysiological changes in calf muscle predict mobility loss at two-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huen R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–380. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. Journal of the American College of Cardiology. 2004;44:618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. Journal of Applied Physiology. 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 10.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. Journal of the American Geriatrics Society. 2006;54:737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 11.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–90. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New England Journal of Medicine. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JMFL, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. NIH Pub. No. 95-4009. [Google Scholar]
- 14.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis and Rheumatism. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 15.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis and Rheumatism. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 16.Ruff CB. Allometry between length and cross-sectional dimensions of the femur and tibia in Homo sapiens sapiens. American Journal of Physical Anthropology. 1984;65:347–58. doi: 10.1002/ajpa.1330650403. [DOI] [PubMed] [Google Scholar]


