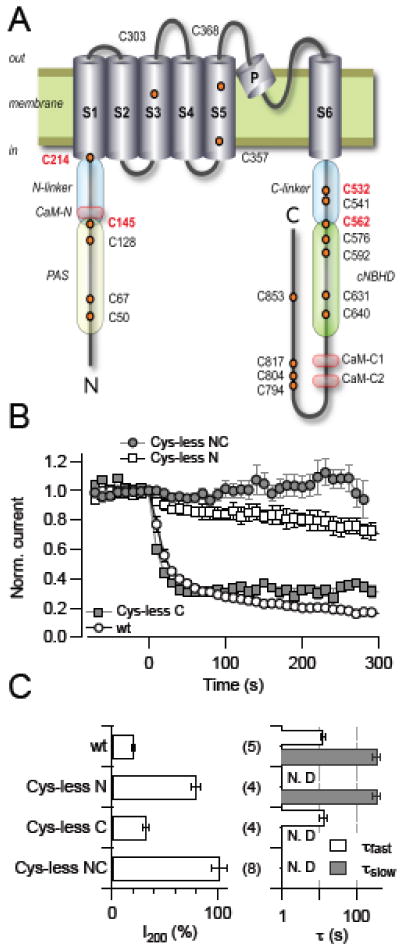
Fig. 4.
Identification of cysteine residues taking part in KCNH1 regulation. (A) Schematic representation of a KCNH1 subunit with the intracellular N terminus, the C terminus, and six transmembrane segments, indicating cysteine residues (orange circles) and regulatory protein domains (CaM, calmodulin binding domains; PAS, Per-ARNT-Sim domain; cNBHD, cyclic nucleotide binding homology domain area). Cysteine residues identified as targets of oxidative channel modification are highlighted in red. Also indicated are the elements connecting the PAS domain and S1 (N-linker) and S6 with the cytosolic C-terminal domain (C-linker). (B) Normalized current for the indicated KCNH1 constructs at 40 mV with DTNB application (20 μM) at time zero. (C) Analysis of the mean remaining current after 200 s DTNB application (I200, left) and up to two time constants of current reduction (right). All data are means ± SEM with n indicated in parentheses. “N.D.” denotes cases in which the corresponding exponential component was not present and, hence, was not determined.