Table 4.
Regioselective cross-couplings of dibrominated p-terphenyls.
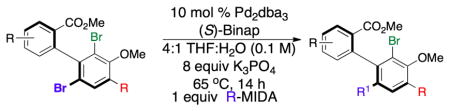 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | % yield (% rec. SM)b | regioselectivityc,d,e | SM er Pdt era |
| 1 | 14b |
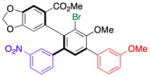 16 |
52 (20) | 10.0:1.0 | 98.0:2.0 |
| 99.0:1.0 | |||||
| 2 | 14b |
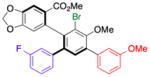 17 |
65 (10) | 5.0:1.0 | 98.0:2.0 |
| 99.0:1.0 | |||||
| 3 | 15c |
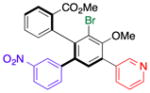 18 |
41 (24) | 7.0:1.0 | 96.0:4.0 |
| 95.0:5.0 | |||||
| 4 | 15c |
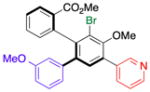 19 |
52 (18) | 6.0:1.0 | 96.0:4.0 |
| 95.0:5.0 | |||||
| 5 | 14h |
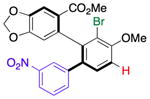 20 |
37 (36) | 2.5:1.0f | 96.0:4.0 |
| 95.0:5.0 | |||||
Enantiomer ratios were determined by chiral HPLC.
Average isolated yield of three experimental runs.
Assigned by NMR analysis.
Ratio determined by 1H NMR.
Averaged over three experimental runs.
Isolated as single regioisomer in 25 % overall yield
