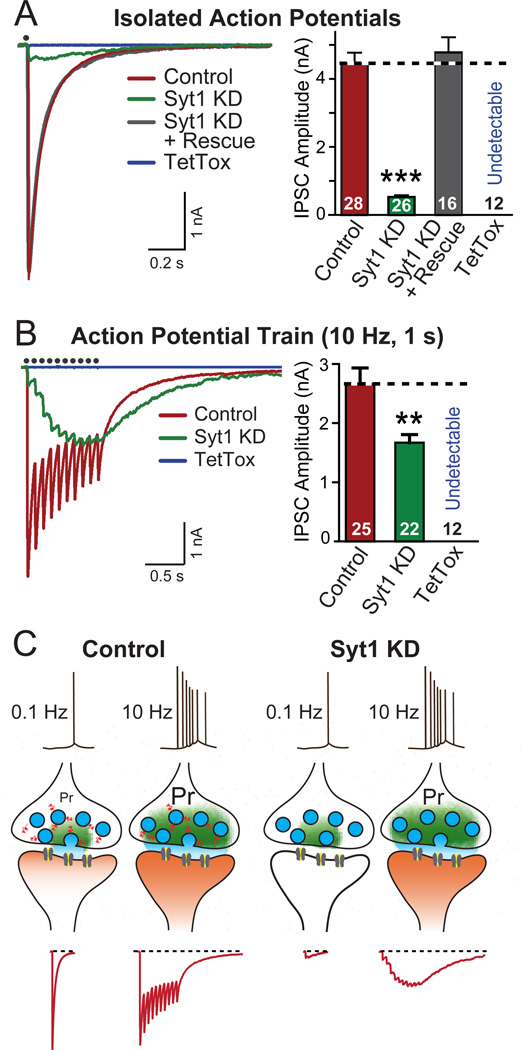
Figure 1. Syt1 KD Effectively Produces a High-pass Frequency Filter for Synaptic Transmission.
(A) Representative traces (left) and summary graphs of amplitudes (right) of IPSCs recorded in cultured neurons infected with lentiviruses expressing mCherry alone (Control), or together with the Syt1 shRNA (Syt1 KD), Syt1 shRNA and Syt1 rescue protein (Syt1 KD + Rescue), or tetanus toxin ligh chain (TetTox). IPSCs were evoked by single-pulse stimuli, and recorded in whole-cell mode (red: control; green: Syt1 KD; gray: Syt1 KD + Rescue; blue: TetTox). Black dots on top of the traces indicate the time when stimulation was delivered.
(B) Same as above, except that IPSCs were evoked by stimulus trains consisting 10 pulses at 10 Hz, and that the summary graphs depict charge transfers during the trains.
(C) Schematic illustration of the synaptic effect of the Syt1 KD. Individual isolated spikes propagate to terminals and trigger Ca2+-dependent neurotransmitter release which is mediated by Syt1 as the Ca2+-sensor. The release probability (Pr) of typical forebrain synapses in response to an individual spike is low. Bursts of spikes increase the overall probability of release by leading to an accumulation of Ca2+, and thereby enhance the reliability of transmission. In Syt1 KD neurons, individual spike-triggered synchronous synaptic release — the majority of synaptic release — is blocked, but the accumulating Ca2+ stimulates unphysiological delayed release that is asynchronous and during longer stimulus trains produces the same overall synaptic transmission as synchronous release, although with a completely different kinetics.
Data in A and B are means ± SEMs; numbers inside columns indicate the number of neurons analyzed in at least three independent experiments. Statistical significance was calculated by Student’s t-test (2-tailed), * p<0.05; ** p<0.01; *** p<0.001. For KD quantitation and additional high-frequency stimulation data, see Fig. S1.