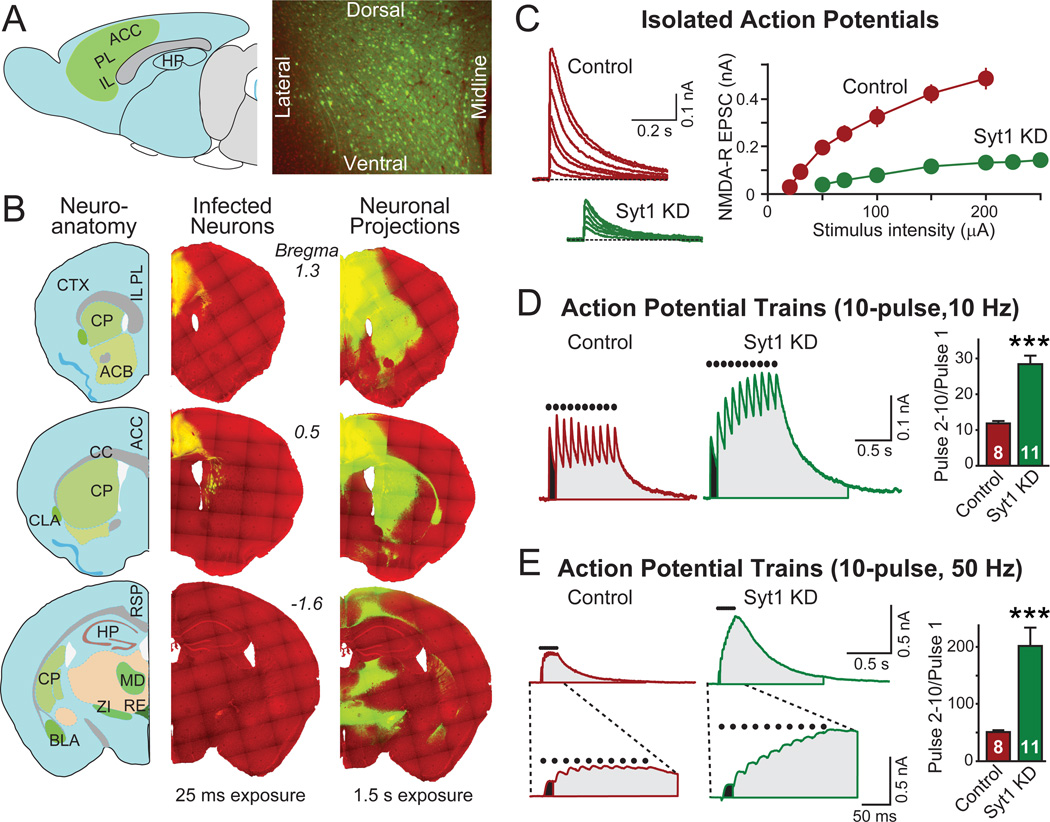
Figure 6. Prefrontal Syt1 KD abrogates synchronous synaptic transmission.
(A) Schematic diagram (left) and documentation of complete neuronal coverage of AAV-mediated gene expression in the prefrontal cortex (right). The green area in the schematic diagram indicates the distribution of AAV infection (ACC: anterior cingulate cortex; PL: prelimbic cortex; IL, infralimbic cortex; HP, hippocampus). The right panel depicts a representative coronal section of the anterior cingulate cortex (green, EGFP; red, DAPI counterstain).
(B) Analysis of the extent of EGFP transport from the medial PFC to other brain areas ~30 days after stereotactic injection of AAVs. An anatomical reference map is depicted on the left, and fluorescence images of coronal brain sections from the same mouse at different anterior-posterior positions are shown on the right with two exposure times (25 ms and 1.5 s; green, EGFP; red, DAPI counterstain). The short exposure detects only EGFP-positive soma, showing their restriction to the prefrontal cortex (a high density of axons in the dorsal striatum is also visible); the long exposure documents the distribution of EGFP-positive axon projections (ACB, nucleus accumbens; BLA, basolateral nucleus of amygdala; CLA, claustrum; CP, caudate putamen; CTX, cortex; MD, mediodorsal nucleus of thalamus; RE, nucleus reunions; RSP, retrosplenial area; ZI, zona incerta).
(C) Input/output curves of excitatory synaptic transmission in the PFC. Representative traces and quantitation of NMDA-receptor mediated EPSCs recorded in acute PFC slices from mice injected with control or Syt1 KD AAV were shown. Whole-cell recordings were obtained in layer2/3 pyramidal neurons; isolated single pulse stimuli were delivered to fibers distributed in layer 1. EPSCs were elicited with increasing stimulus strength (control, n=7; Syt1 KD, n=11).
(D, E) Representative traces and quantitation of NMDA receptor-mediated EPSCs in acute PFC slices showing increased facilitation in response to stimulus trains in Syt1 KD. EPSCs were elicited by trains of stimuli at 10 Hz (E) or 50 Hz (F); dots on top of the traces indicate the time points of stimulation, the charge transfer during pulse 1 is colored black, and the charge transfer during subsequent pulses gray. The bottom traces in F depict expansions of the top traces to illustrate the EPSC kinetics. Facilitation (the pulse 2–10/pulse 1 or pulse 2–5/pulse 1 ratio) was calculated as the ratio of the charge transfer induced by the indicated pulses (right bar diagram). Since prefrontal neurons receive excitatory inputs from various brain regions besides the infected prefrontal neurons, the impact of TetTox or of the Syt1 KD on synaptic transmission is likely underestimated (control, n=8; Syt1 KD, n=11).
Data are means ± SEM; numbers inside or on top of columns indicate number of neurons analyzed. Statistical significance between groups (comparing Syt1 KD or TetTox to control) was determined with student’s T-test (*** p<0.001). For more information on prefrontal infection, see Figure S4.