Figure 1.
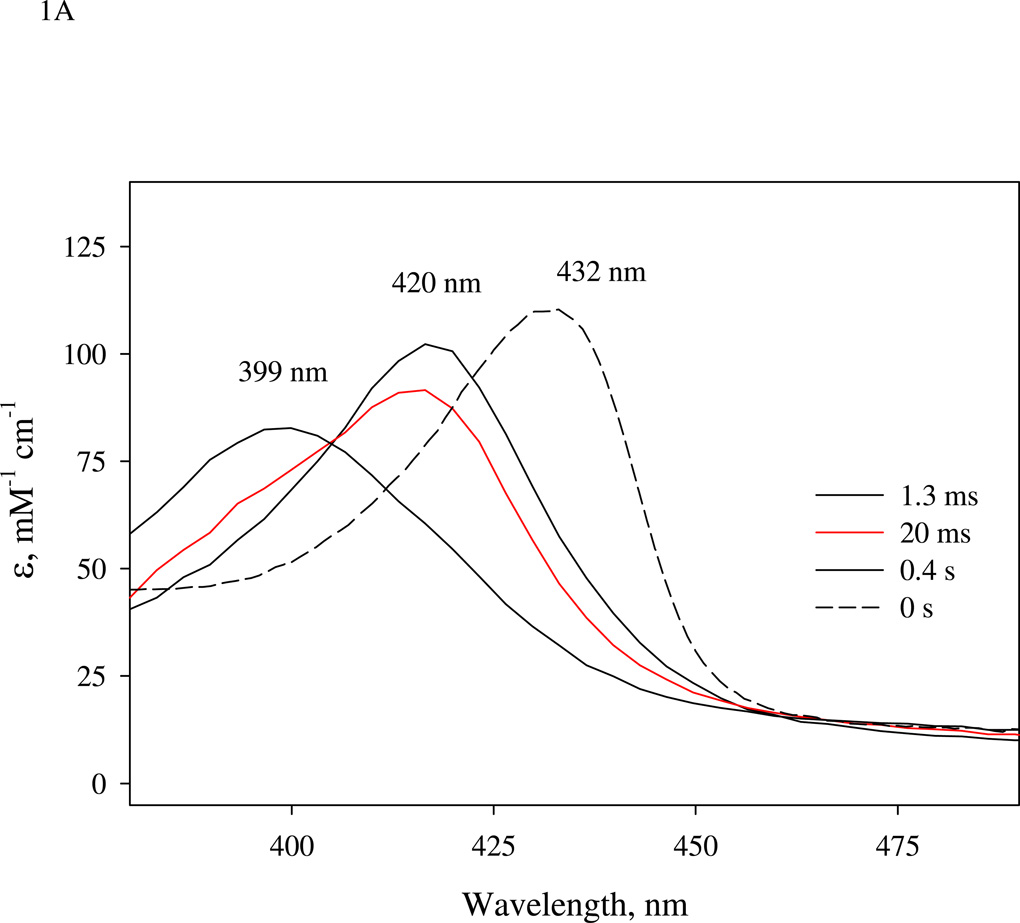
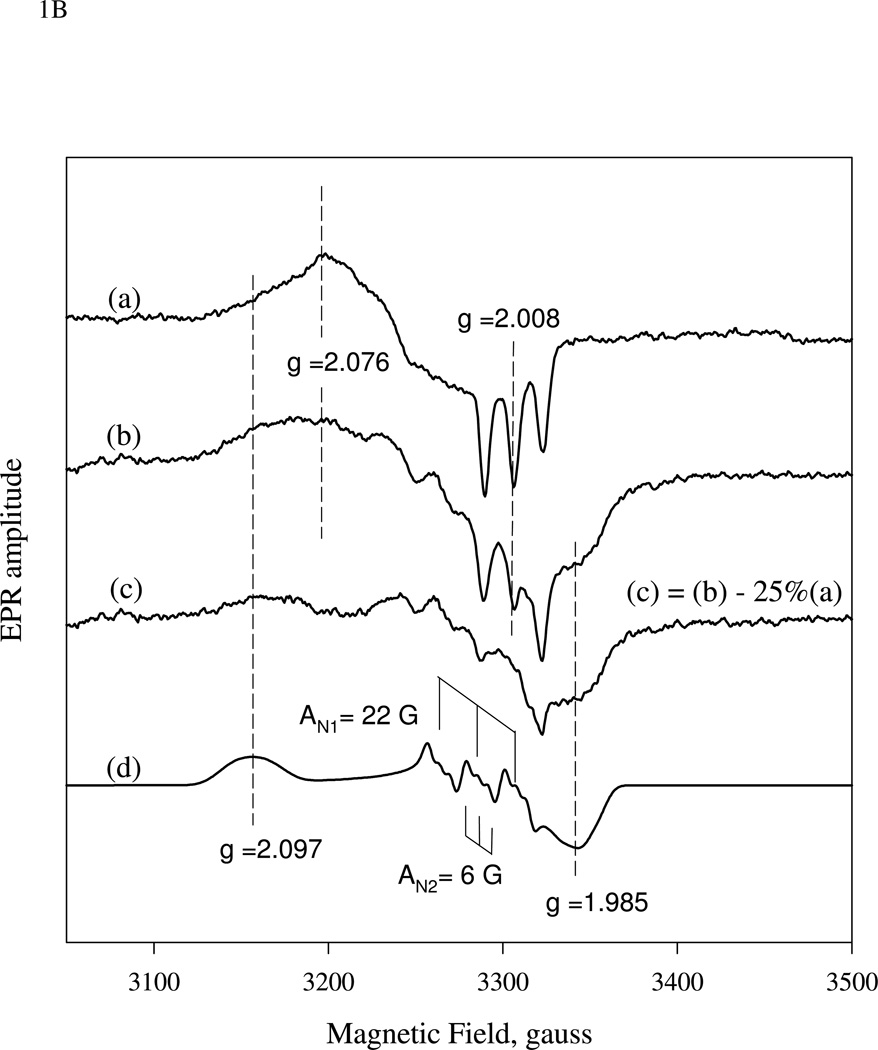
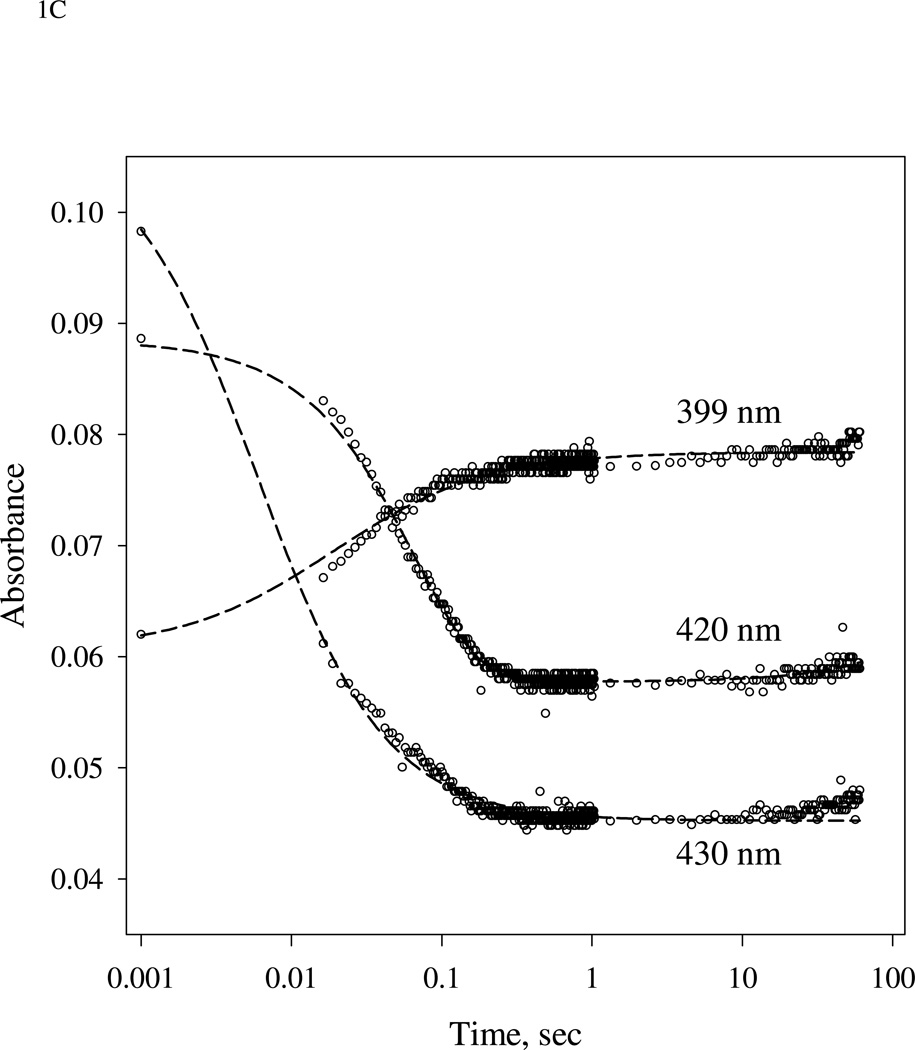
(A).Time-resolved spectra of the reaction between sGC and stoichiometric amount of NO. NO binding reactions of sGC monitored by rapid-scan single mixing at 24 °C. Both sGC and NO were at 7 µM. Three spectra recorded at 1.3 ms, 20 ms and 1.0 s out of the 400 collected are shown. A matching resting Fe(II) sGC spectrum collected on a HP8453 spectrophotometer is also shown as "zero" time control (dashed line). (B). Transient formation of 6c NO-sGC identified by RFQ EPR kinetic measurement. 10 µM each of sGC and NO was reacted at 24 °C by single-stage equal mixing and freeze trapped by cold isopentane (113 K). The ram velocity was 1.25 cm/s and the packing factor was 0.45 (16). The EPR spectra for samples trapped at 20 ms, (b), and 5 s, (a) are one out of three repeats, which show similar results. Spectrum (c) is the difference between (b) and 25% of (a) to reveal the dominant 6c NO-sGC species. Simulation of the EPR of 6c NO-sGC, spectrum (d), was done by Simfonia as frozen powder sample. Hyperfine splitting constants related to the nitrogen nuclei from NO, AN1, and His105, AN2, are indicated. Spectra (a) and (b) are the average of 32 scans. Key g values for the NO-sGC complexes are also indicated. (C). Kinetics of the resting sGC (by A432 nm), 6c NO-sGC (A420 nm) and 5c NO-sGC (A399 nm) in the second stage reaction of the anaerobic sequential stopped flow at 24 °C. Reaction in the first stage was between 5 µM sGC and 5 µM NO, and the mixture, after 20 ms delay, was further reacted with 5 µM NO in the 2nd mixing. Dashed lines are visual guide connecting all data points which are not evenly distributed in logarithmic scale, especially those < 10 ms.