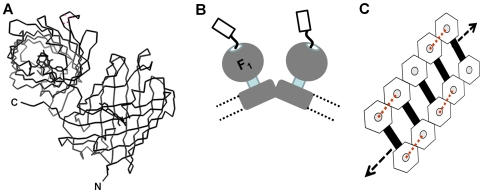
Figure 1. Using HcRed to crosslink ATP synthase complexes in the mitochondrial inner membrane.
A. Fluorescent HcRed is an obligate homo-dimer. Representation shown in ribbon format was generated using PDB, 1YZW; [5]. The N- and C- termini are labelled for one of the two protomers. HcRed fused at its N-terminus to subunit γ of mtATPase promotes cross-linking of neighbouring mtATPase dimers in the membrane. B. Cartoon based on reported EM data showing for the inner membrane of the mitochondrion, the relative arrangement of each mtATPase monomer in dimer pairs. The C-terminus of subunit γ fused via a 25 amino acid polypeptide linker to an HcRed protomer is shown extending through the central pit of the F1 - sector and projecting into the matrix space. The distance between F1-sectors in these dimer pairs is too large for HcRed itself to form fluorescent dimers with a linker of this length. Broken lines represent the membrane boundaries. C. Dimer pairs of mtATPase embedded in the inner-membrane of the mitochondrion are organised into extended higher order assemblies resembling a ribbon. This organisation is important for proper cristae formation. Observed from the matrix and perpendicular to the inner membrane the catalytic sector which projects into the matrix space is represented by the hexagon shape from which the C-terminus of the γ-subunit protrudes from a central pit linked to an HcRed protomer (grey circle). Links at the level of the membrane between mtATPase complexes to form dimer pairs are shown by the black bar. The length of the linker between HcRed and subunitγ is sufficient to allow cross-links (rd dotted lines) to form between neighbouring dimers of mtATPase complexes, but not within dimer pairs.