Table 1.
Catalytic phosphitylation and deoxygenation of alcohol substrates.
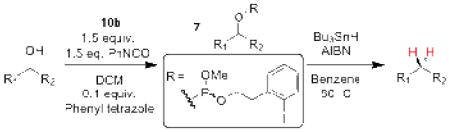 | |||
|---|---|---|---|
| Entry | Alcohol | Phosphite | Deoxygenated Product |
| 1 |
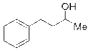 11 |
 13 >95% NMR Conversion |
Not Determined |
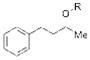
| 2 |
 12 |
14 >95% NMR Conversion |
Not Determined |
| 3 |
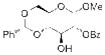 15 |
Not Isolated |
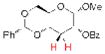 18, 77% yielda |
| 4 |
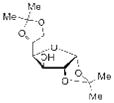 16 |
Not Isolated |
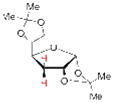 19 68% yielda |
| 5 |
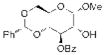 17 |
Not Isolated |
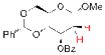 20 67% yielda |
| 6 |
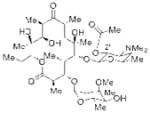 2′-Acetyl Ery A |
21 (4″-Phosphite) 83% isolated yield |
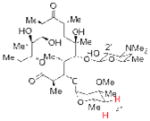 22 4″-deoxy Ery A 84% yieldb |
Isolated yields of the deoxygenated species are given for the two-step process.
Isolated yield determined from phosphite 21 after methanol cleavage of 2′-acetyl protecting group.
