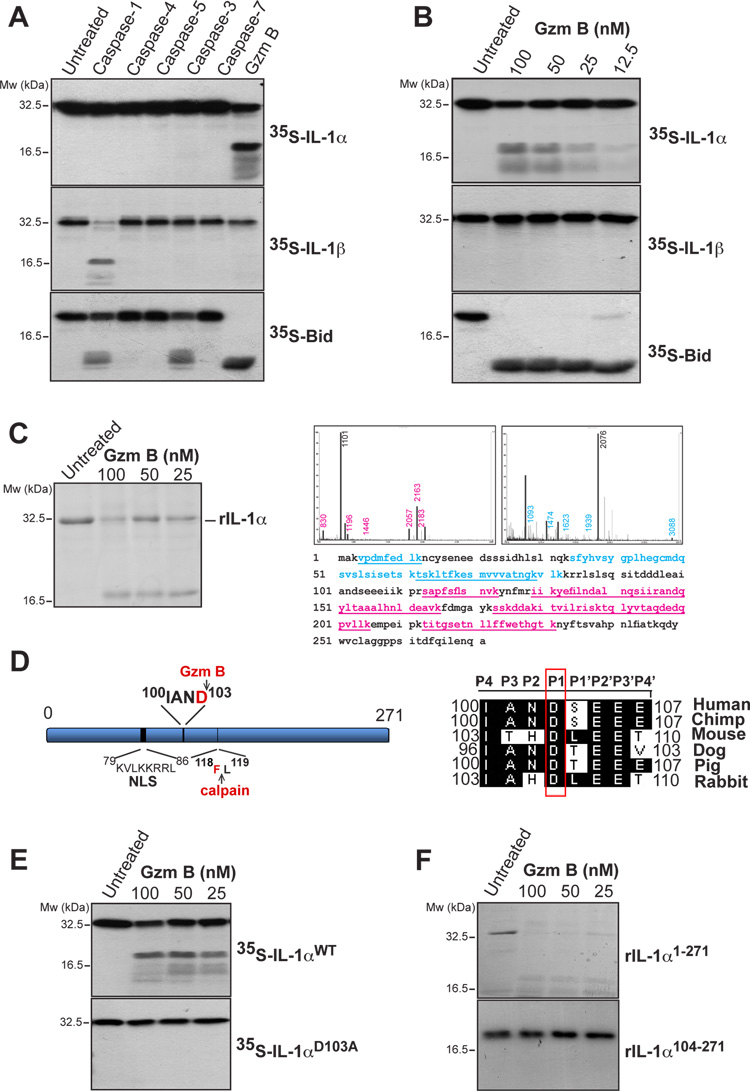
Figure 1. IL-1α is a substrate for granzyme B.
(A) 35S-labeled IL-1α, IL-1β and Bid were incubated at 37°C for 2 h, either alone, or in the presence of recombinant caspase-1, -4, -5 (20 nM), caspase- 3, -7 or granzyme B (200 nM) followed by analysis by SDS-PAGE/fluorography.
(B) 35S-labeled IL-1α, IL-1β and Bid were incubated for 2 h at 37°C with the indicated concentrations of granzyme B and analysed as in (A).
(C) Recombinant purified full length IL-1α was incubated at 37°C for 2h with the indicated concentrations of granzyme B and analysed by SDS-PAGE with Coomassie staining. IL-1α cleavage fragments were excised from the gel and analysed by MALDI-TOF mass spectrometry. Mass spectra of cleavage fragments are indicated, with coverage of the two cleavage fragments underlined in blue and red, respectively.
(D) Schematic representation of IL-1α indicating nuclear localisation signal and granzyme B and calpain-1 cleavage sites. A sequence alignment of the putative granzyme B cleavage site in IL-1α from a number of mammals is shown to the right. The P1 Asp residue is indicated.
(E) 35S-labeled IL-1αWT and IL-1αD103A mutant were incubated at 37°C for 2h with the indicated concentrations of granzyme B followed by analysis by SDS-PAGE/fluorography.
(F) Recombinant IL-1αFL and IL-1α104-271 were incubated for 2 h at 37°C with the indicated concentrations of granzyme B, then analysed by SDS-PAGE and Coomassie staining. All data shown are representative of at least three independent experiments.