Table 2.
Pd-catalyzed carboetherification reactions of phenols bearing pendant alkenesa
| Entry | Substrate | RBr | Product | Yieldb |
|---|---|---|---|---|
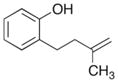
| 1 |
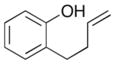 7 |
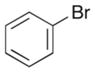
|
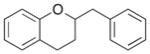 8 |
75% |
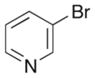
| 2 | 7 |
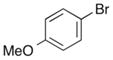
|
14 |
57% |
| 3 | 7 |
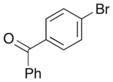
|
 15 |
51% |
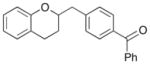
| 4 |
 9 |
|
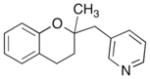 16 |
87% |
| 5 | 9 |
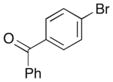
|
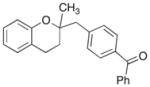 17 |
71% |
| 6 | 9 |
|
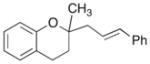 18 |
83% |
| 7 | 9 |
|
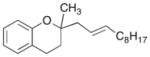 19 |
68% |
| 8 |
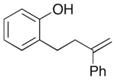 10 |
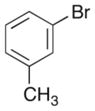
|
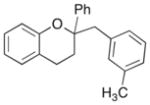 20 |
54% |
| 9 | 10 |
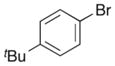
|
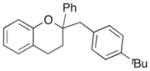 21 |
56% |
| 10 |
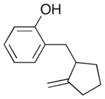 11 |
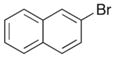
|
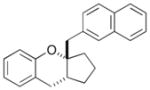 22 |
59% >20:1 dr |
| 11 |
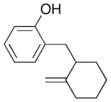 12 |
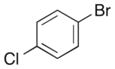
|
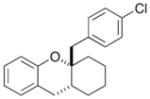 23 |
71% >20:1 dr |
| 12 | 12 |
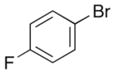
|
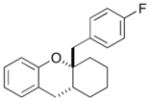 24 |
63% >20:1 dr |
| 13 |
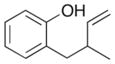 13 |
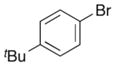
|
25 |
ca. 47%c 2:1 dr |
| 14 |
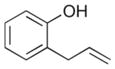 5 |
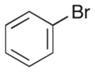
|
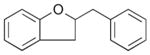 26 |
37% |
Conditions: 1.0 equiv phenol substrate, 2.0 equiv R–Br, 2.0 equiv NaOtBu, 2 mol % Pd2(dba)3, 4 mol % S-Phos, toluene (0.25 M), 110 °C.
Isolated yield (average of two experiments).
The two inseparable stereoisomeric products were isolated in 63% yield and ca. 75% purity. The remaining 25% of the mixture was primarily composed of an unidentifed low molecular weight side product that appears to be derived from the phenol substrate.
