FIGURE 1. RT1-A1c has a preference for binding B7-supertype peptides.
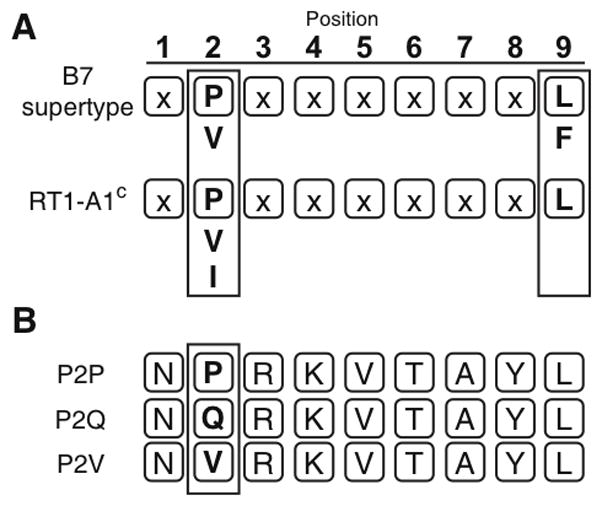
A. The HLA-B7 supertype (top) and RT1-A1c (bottom) peptide anchor residue binding preferences are shown (boxed). Both HLA-B7 and RT1-A1c have preference for proline at the P2 position and have leucine as a preferred amino acid residue at the C-terminus. Secondary anchor residues appear below primary anchor residues. The B7 supertype has a more variable amino acid binding at the C-terminus (A,L,I,V,M,F,W,Y), but only the binding preference for the prototypical member, HLA-B7, is shown for simplicity. B. Three nonamer peptides were used in this study. P2P is the “ideal” peptide sequence previously reported (12). P2Q and P2V have the same sequence, except glutamine or valine has been substituted at the P2 position.
