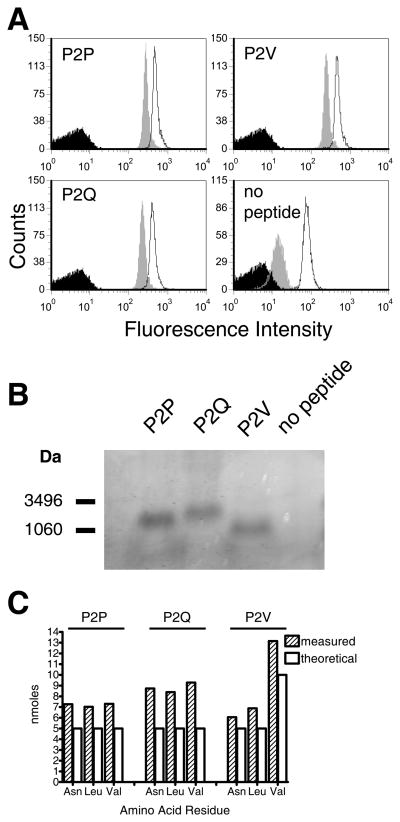
FIGURE 3. Conformational integrity, thermal stability and peptide content of refolded RT1-A1c.
A. Conformational integrity and thermal stability analysis. RT1-A1c refolded with P2P, P2Q, P2V, or no peptide was captured onto avidin-coated 5 μm latex beads and incubated for 1 hr at 37°C (grey histograms) or 4°C (open histograms) followed by FACS analysis with the RT1-A1c specific Ab, YR5/12. Avidin beads with no protein captured were stained with antibodies in parallel (black histograms). B. The presence of P2P, P2Q, or P2V peptide in the refolded and purified RT1-A1c was analyzed by tricine SDS-PAGE. Approximately 15 μg of purified RT1-A1c was denatured in sample buffer and run on a 16.5% Tricine-SDS polyacrylamide gel. C. Peptide amino acid quantification. Five nmoles of each refolded RT1-A1c–peptide complex were treated with acid to separate the peptide as described in the Materials and Methods. Eluted peptides were subjected to acid hydrolysis and the amino acids quantified.