Abstract
Purpose
Although the incidence of gastric cancer has declined in the general population, it is the second most frequent cause of death due to malignancy in the world with its incidence in the elderly increasing as a result of increased life expectancy. This present study tried to find the optimal treatment for patients aged 75 years or older with gastric cancer through comparison of the clinicopathological characteristics, surgical outcomes, and identifying prognostic factors of survival.
Methods
Elderly patients who underwent gastric resection for gastric cancer from January, 1999 to February, 2009 (n = 470) were divided into two groups: very elderly patients, 75 years or older (n = 95), and younger elderly patients, between 65 and 74 years old (n = 365).
Results
Distinct characteristics of very elderly patients included more frequent underlying disease, deeper invasion, and more frequent lymph node metastasis. There were significant differences in overall survival between the two groups at stages III-B and IV. However, postoperative hospital stays, postoperative morbidity, mortality and early stage did not differ between curatively resected patients in the two groups.
Conclusion
Due to improved postoperative care, gastrectomy of gastric cancer is the treatment of choice in very elderly patients. Therefore, early diagnosis through regular medical screening and curative gastrectomy with lymph node dissection should be performed in very elderly gastric cancer patients.
Keywords: Elderly, Gastric cancer, Geriatric surgery
INTRODUCTION
Gastric cancer is one of the most common cancers in the world. Curative treatment of stomach cancer requires gastric resection [1], and surgical approaches to treat potentially curable gastric cancers, including extended lymphadenectomy, yield better results than less radical procedures [2]. The World Health Organization defines people 65-and-over as senior citizens. Globally, the number of people in this age group is increasing at an unprecedented rate, and most of this increase is occurring in developed countries [3,4]. Although the incidence of gastric cancer has declined in the general population, it is the second most frequent cause of death due to malignancy in the world, and its incidence in the elderly is increasing as a result of increased life expectancy. Elderly people are sometimes divided into the young-old (65 to 74 years), the old-old (75 to 84 years) and the oldest-old (85+ years) [5]. In Korea, many old-old and oldest-old persons are older than the age of average human life expectancy (male 76, female 83). Although gastric surgery has become more common for young-old patients, surgeons often hesitate to perform operations on the old-old and the oldest-old patients. As many old-old and oldest-old patients have comorbidities, the risk of surgery is often higher. In some elderly patients, gastric cancer may not affect life expectancy because the patient has already some lethal comorbidity. Therefore it is important to evaluate clinicopathological characteristics and surgical outcomes, including postoperative morbidity and mortality, in the old-old and oldest-old patients. Some studies have compared outcomes between elderly and young patients with gastric cancer [6,7]. However, gastric cancer in young patients also has distinctive properties. So, this present study tried to find the optimal treatment of patients who ages of 75 years or older with gastric cancer through comparison of the clinicopathological characteristics, surgical outcomes, and identifying prognostic factors of survival.
METHODS
A total of 470 patients with histologically confirmed primary gastric cancer who had underwent gastrectomy between January, 1999 and February, 2009 at the Department of Surgery were enrolled in this study. These patients included 95 old-old and oldest-old patients (age of 75 years or older) and 375 young-old patients (65 to 74 years old). The present study defined "very elderly" patients as those aged 75 or older, according to criteria used in previously published literature [5]. Four hundred and forty-one patients underwent curative resection (86 vs. 355) and 29 patients were treated with palliative resection (9 very elderly patients and 20 young-old patients). Preoperative studies to determine the location of the tumor, macroscopic appearance, depth of invasion, and lymph node and distant metastasis were routinely performed using gastroscopy and computed tomography. The surgical procedure was considered curative (R0) if gross tumor tissue, including metastatic lymph nodes, was removed completely and the microscopic examination revealed no cancer cells at both proximal and distal margins. The procedure was considered palliative (R1 and R2) if macroscopic or microscopic disease was left behind. The Japanese Gastric Cancer Association has standardized lymph node dissection for gastric cancer [8]. In the present study, lymph node dissection (D2 or D3) was performed in accordance with the guidelines of the Japanese Gastric Cancer Association. All patients were followed up according to our standard protocol, which includes tumor markers, gastroscopy, computed tomography and chest radiography. The median follow-up period was 28 months and data were analyzed with IBM SPSS ver 18.0 (IBM Co., New York, NY, USA). Sixteen clinicopathological variables were used for statistical analysis: patient age, gender, family history of cancer, underlying disease, preoperative symptoms, synchronous metastasis, method of operation, curability, postoperative hospital stay, location of tumor, histological type, depth of invasion, lymph node metastasis, lymphatic invasion, venous invasion, and pathologic stage. Patient characteristics were compared using Fisher's exact tests, chi-square tests, Mann-Whitney U tests and analysis of variance. The Cox proportional hazards regression model was used to identify prognostic factors. Overall survival was calculated by Kaplan-Meier estimation and examined by log-rank tests. P-values were considered statistically significant at the 0.05 level.
RESULTS
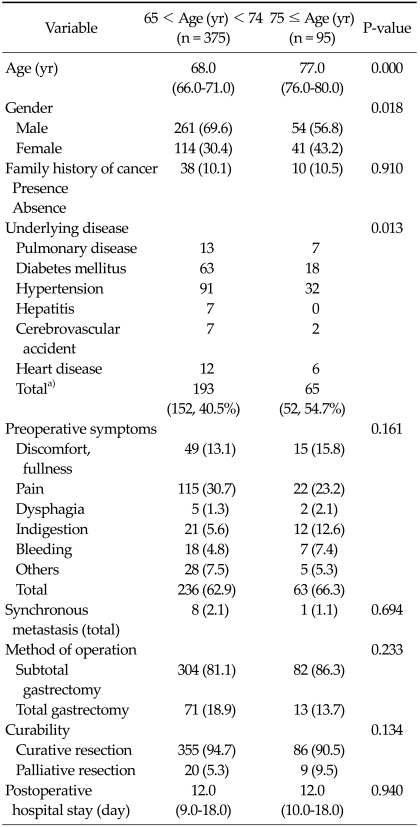
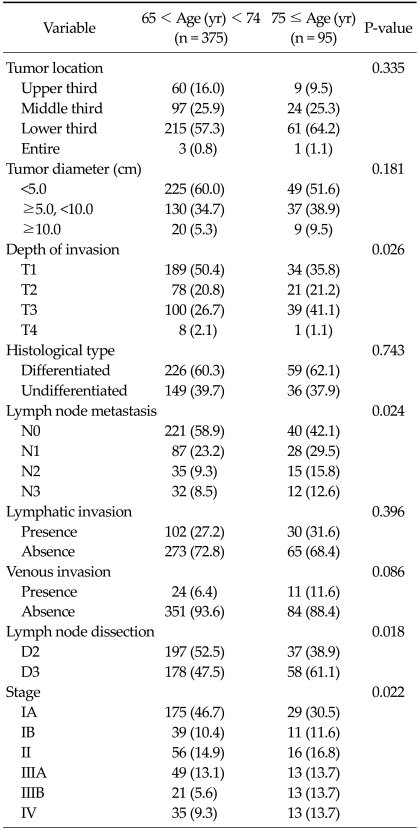
The clinicopathological features of all 470 registered gastric cancer patients are listed in Tables 1,2. There were significant differences in gender, underlying disease, depth of invasion, lymph node metastasis, lymph node dissection, and pathological stage between the two groups. In very elderly patients, female gender and underlying disease were significantly more frequent. Among the pathological characteristics, depth of invasion and lymph node metastasis and pathological stage were higher among very elderly patients. In the post hoc analysis, T3 of depth invasion (P = 0.003), N1 and N2 of lymph node metastasis (P = 0.036 and P = 0.013), and pathological stage IIIB, IV (P = 0.001 and P = 0.031) were significantly more frequent than T1, N0, IA in very elderly patients. In the method of operation, the ratio of operation type did not differ between subtotal gastrectomy and total gastrectomy (P = 0.233), but lymph node dissection of D3 was significantly more frequent in very elderly patients (P = 0.018). Among the underlying diseases of the two age groups (very elderly patients, 65 cases and 52 patients; young-old patients, 193 cases and 152 patients), hypertension was the most frequent, followed by diabetes mellitus, and pulmonary disease (P = 0.013). However, in the preoperative symptoms of the two age groups, abdominal pain was most frequent, followed by fullness, but the difference was not significant.
Table 1.
Characteristics of patients undergoing resection
Values are presented as median (range) or number (%).
a)Values are presented as no. of complicated cases (no. of complicated patients, %).
Table 2.
Pathological results
Values are presented as number (%).
Application of 1997 Union for International Cancer Control/American Joint Committee on Cancer 6th tumor-node-metastasis classification.
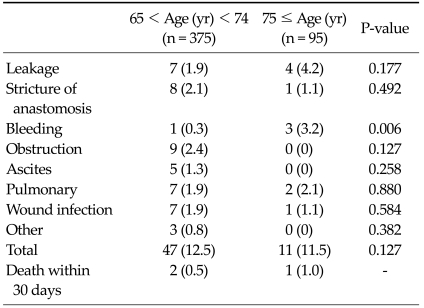
Among all registered patients, postoperative complications were observed in 58 patients (12.3%) (11.5% of very elderly vs. 12.5% of young-old patients, P = 0.127). The incidence of bleeding was significantly higher in very elderly compared to young-old patients. However, there was no significant difference for other complications (Table 3). Postoperative mortality was observed in 3 patients (0.6%) (1.0% of very elderly patients vs. 0.5% in young-old patients) but the difference was not significant.
Table 3.
Postoperative morbidity and mortality
Values are presented as number (%).
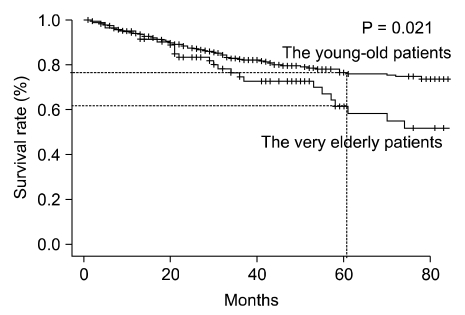
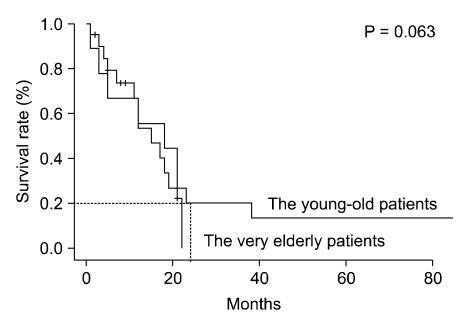
Among the resected patients, there was a significant difference in overall survival (OS) rate between two groups (5-year survival, 55.9% vs. 73.5% respectively; P = 0.006). In addition, among the curatively resected patients, there was a significant difference in OS between two groups (5-year survival, 61.5% vs. 76.5% respectively; P = 0.021) (Fig. 1). However, among the palliatively resected patients, there was no significant difference in OS between two groups (2-year survival, 0.0% vs. 20.0% respectively; P = 0.063) (Fig. 2).
Fig. 1.
Survival of very elderly patients (≥75 years) and younger elderly patients (65 to 74 years) after curative resection. There were significant differences in overall survival between the two age groups (P = 0.021).
Fig. 2.
Survival of very elderly patients (≥75 years) and younger elderly patients (65 to 74 years) after palliative resection. There was no significant difference in overall survival between the two age groups (P = 0.063).
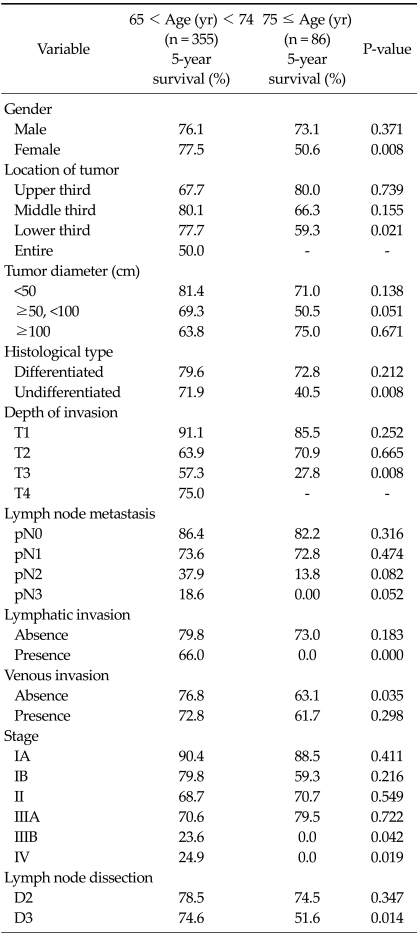
OS rates in the patients who underwent curative gastrectomy according to clinicopathological variables are presented in Table 4. There were significant differences between two groups in 5-year survival rate; sex, tumor location, histological type, depth of invasion, lymphatic invasion, and venous invasion. For each of these variables, the 5-year survival rate was significantly worse in very elderly patients. In patients receiving curative gastrectomy, the OS rate was significantly worse among the very elderly than among the young-old patients for stages IIIB (P = 0.042) and IV (P = 0.019) (Table 4). However, OS rates for stage I, II, IIIA did not differ significantly between the two age groups.
Table 4.
Five-year survival rates according to clinicopathological variables in patients undergoing curative resection
Application of 1997 Union for International Cancer Control/American Joint Committee on Cancer 6th tumor-node-metastasis classification.
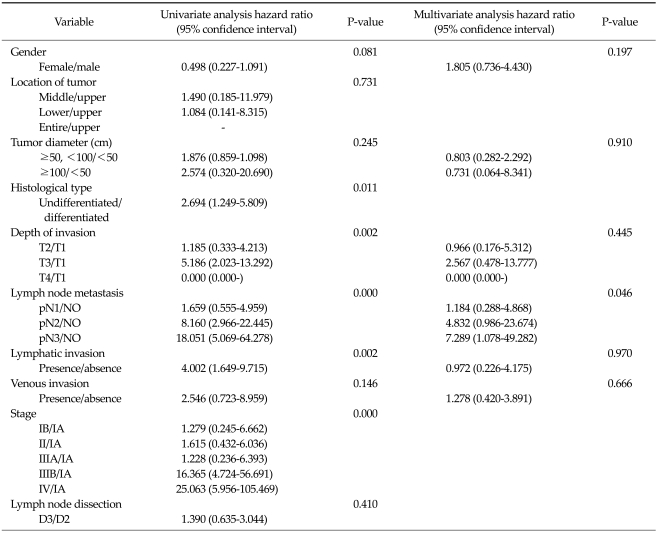
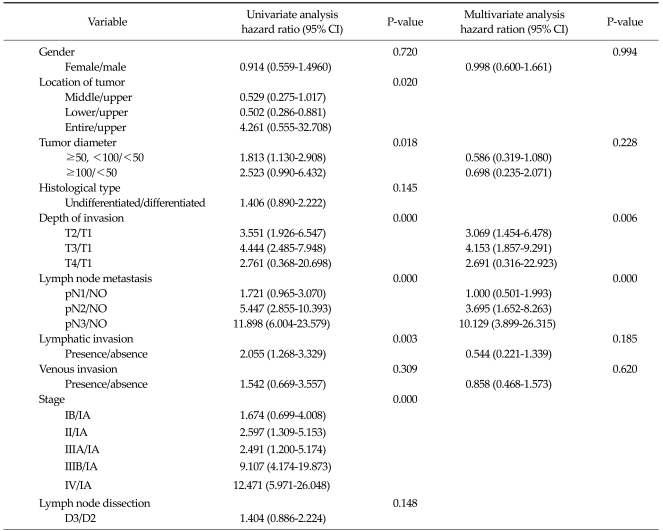
In very elderly patients receiving curative gastrectomy, histological undifferentiated type, depth of invasion, lymph node metastasis, and lymphatic invasion significantly affected prognosis according to univariate analysis, and lymph node metastasis independently influenced prognosis according to multivariate analysis (Table 5). In young-old patients receiving curative gastrectomy, the location of the tumor, tumor diameter, depth of invasion, lymph node metastasis, and lymphatic invasion significantly affected the prognosis according to univariate analysis, and depth of invasion and lymph node metastasis independently influenced prognosis in this patient group by multivariate analysis (Table 6).
Table 5.
Cox proportional hazards regression model in patients ≥75 years of age undergoing curative resection
Application of 1997 Union for International Cancer Control/American Joint Committee on Cancer 6th tumor-node-metastasis classification.
Table 6.
Cox proportional hazards regression model in patients 65 to 74 years of age undergoing curative resection
CI, confidence interval.
DISCUSSION
Average life expectancy in Korea has progressively increased because of better living conditions, increased consumption of nutritious foods including fruits and vegetables, and improved treatment of comorbidities and other diseases [9,10]. The incidence of gastric cancer as well as other cancers has increased in elderly patients. Moreover, the increased age of the population is accompanied by an increase in age-related diseases. Preoperative surgical risk is often higher in elderly patients than young patients [11-13]. Therefore it is important to develop therapeutic strategies for elderly patients with gastric cancer.
The average age of gastric cancer patients in this study is 70.5 ± 4.6 years. In the present study, elderly patients were divided into two groups, a young-old group and a very elderly group. The definition of very elderly patients (age of 75 years or above) used in this study was suitable to obtain a sufficient sample size to attain statistical significance.
In the present study, very elderly patients show distinctive characteristics including higher ratio of female gender and more frequent underlying disease than young-old patients. Finding of more frequent underlying disease is consistent with those findings of other report [11]. However, the ratio of females is not consistent with the results of other reports [14,15]. Other reports showed that female had a lower ratio in old age (Young age male:female vs. old age is approximately 1:1 vs. 2:1 or 2:1 vs. 2:1) than this present study (approximately 2:1 vs. 1:1).
Generally, differentiated type of gastric carcinoma located in the lower third of the stomach in the elderly originates from intestinal metaplasia because of atrophic gastritis induced by exogenous factors [16], or from Helicobacter pylori infection [17,18]. In pathological results, the tumor is most frequently located in the lower third and histological differentiated type is dominant without a significant difference in both age groups. The results obtained in the present study are compatible with these previous observations [16-18]. However, there were significant differences in the depth of invasion and lymph node metastasis between the two groups. Very elderly patients had deeper invasion and more advanced lymph node metastases than young-old patients (P = 0.024). Previous studies reported that 51% and 45% of elderly patients had stage III-IV gastric cancer and that there were no significant differences between the two age groups (around 80 or 75 years old, respectively) [19,20]. However, in the present study, although very elderly patients had a similar ratio (41.1%) of stage III and IV as previous studies, there are significant differences between the two groups. Very elderly patients had more advanced gastric cancer in ratio than young-old patients. The main reason for the poor prognosis in very elderly patients was the low proportion of early stage tumors and the high proportion of advanced stage tumors with statistical significance (P = 0.022). This may be related to the tendency to ignore preoperative symptoms and low rates of medical screening by endoscopic examination in very elderly patients [21-23].
Although D3 lymph node dissection was more frequent in very elderly patients with a similar ratio of method of operation and curability (R0), the incidence of total postoperative morbidity, mortality and postoperative hospital stay did not show a significant difference. In patients receiving D3 lymph node dissection, very elderly patients showed a lower 5-year survival rate compared to youn-gold patients, but it had no effect as a prognostic factor. And if a more advanced pattern in very elderly patients is considered, a low 5-year survival rate is a reasonable result. Previous other studies show higher morbidity (29%, 33%) and mortality (3%, 8%) than in the present study [19,23]. However, the differences were not statistically significant in previous other studies, either. Advances in surgical techniques, postoperative intensive care, and improved performance of very elderly patients contributed to the similarity in incidence of surgical morbidity and mortality in the two age groups.
Although prognostic factors were similar in the two groups, the overall 5-year survival rate was significantly different in the two groups receiving curative gastrectomy. However, when an exhaustive inspection of results was carried out, earlier stages (I-IIIA) have no significant differences and more advanced stages (IIIB-IV) have significant differences in overall 5-year survival rate. In several previous studies, the 5-year survival rate was significantly different between the two age groups; but when the mortality for gastric cancer alone was considered, there was no difference [20,24]. However, a Dutch D1-D2 study reports that the 5-year survival rates of elderly patients are worse than in people younger than 65 years, not only considering all causes of death, but also when death from gastric cancer is evaluated alone [25]. These constitute limitations of the present study. Additional studies would benefit from research into real causes of death and comparisons of disease specific survival in the two groups.
In conclusion, very elderly patients can recover from aggressive gastric cancer surgery without increases in postoperative morbidity and mortality with advanced postoperative intensive care. The long term survival rates of very old patients do not differ from those of young-old patients, especially in early stage disease (I, II, IIIA). Therefore, early diagnosis through regular medical screening and curative gastrectomy with lymph node dissection should be performed in very elderly gastric cancer patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 2.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. The Surgical Cooperative Group. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 3.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 4.Monson K, Litvak DA, Bold RJ. Surgery in the aged population: surgical oncology. Arch Surg. 2003;138:1061–1067. doi: 10.1001/archsurg.138.10.1061. [DOI] [PubMed] [Google Scholar]
- 5.Crews DE, Zavotka S. Aging, disability, and frailty: implications for universal design. J Physiol Anthropol. 2006;25:113–118. [PubMed] [Google Scholar]
- 6.Fujimoto S, Takahashi M, Ohkubo H, Mutou T, Kure M, Masaoka H, et al. Comparative clinicopathologic features of early gastric cancer in young and older patients. Surgery. 1994;115:516–520. [PubMed] [Google Scholar]
- 7.Nakamura T, Yao T, Niho Y, Tsuneyoshi M. A clinicopathological study in young patients with gastric carcinoma. J Surg Oncol. 1999;71:214–219. doi: 10.1002/(sici)1096-9098(199908)71:4<214::aid-jso2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 9.McKenna RJ., Sr Clinical aspects of cancer in the elderly. Treatment decisions, treatment choices, and follow-up. Cancer. 1994;74(7 Suppl):2107–2117. doi: 10.1002/1097-0142(19941001)74:7+<2107::aid-cncr2820741719>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita I, Hanai H, Kajimura M, Tamakoshi K, Nakajima T, Matsubayashi Y, et al. Should gastric cancer patients more than 80 years of age undergo surgery? Comparison with patients not treated surgically concerning prognosis and quality of life. J Clin Gastroenterol. 2002;35:29–34. doi: 10.1097/00004836-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Katai H, Sasako M, Sano T, Maruyama K. The outcome of surgical treatment for gastric carcinoma in the elderly. Jpn J Clin Oncol. 1998;28:112–115. doi: 10.1093/jjco/28.2.112. [DOI] [PubMed] [Google Scholar]
- 12.Houry S, Amenabar J, Rezvani A, Huguier M. Should patients over 80 years old be operated on for colorectal or gastric cancer? Hepatogastroenterology. 1994;41:521–525. [PubMed] [Google Scholar]
- 13.Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY, P'eng FK. Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg. 2000;24:465–472. doi: 10.1007/s002689910074. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Yamane T, Sawai K, et al. Clinicopathological characteristics of gastric cancer in the elderly. Br J Cancer. 1996;73:798–802. doi: 10.1038/bjc.1996.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol. 2005;11:22–26. doi: 10.3748/wjg.v11.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 17.Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991;83:640–643. doi: 10.1093/jnci/83.9.640. [DOI] [PubMed] [Google Scholar]
- 18.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 19.Orsenigo E, Tomajer V, Palo SD, Carlucci M, Vignali A, Tamburini A, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer. 2007;10:39–44. doi: 10.1007/s10120-006-0409-0. [DOI] [PubMed] [Google Scholar]
- 20.Coniglio A, Tiberio GA, Busti M, Gaverini G, Baiocchi L, Piardi T, et al. Surgical treatment for gastric carcinoma in the elderly. J Surg Oncol. 2004;88:201–205. doi: 10.1002/jso.20153. [DOI] [PubMed] [Google Scholar]
- 21.Trias M, Targarona EM, Ros E, Bordas JM, Perez Ayuso RM, Balagué C, et al. Prospective evaluation of a minimally invasive approach for treatment of bile-duct calculi in the high-risk patient. Surg Endosc. 1997;11:632–635. doi: 10.1007/s004649900409. [DOI] [PubMed] [Google Scholar]
- 22.Bergman JJ, Rauws EA, Tijssen JG, Tytgat GN, Huibregtse K. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile duct stones: report on 117 patients. Gastrointest Endosc. 1995;42:195–201. doi: 10.1016/s0016-5107(95)70091-9. [DOI] [PubMed] [Google Scholar]
- 23.Saidi RF, Bell JL, Dudrick PS. Surgical resection for gastric cancer in elderly patients: is there a difference in outcome? J Surg Res. 2004;118:15–20. doi: 10.1016/S0022-4804(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 24.Eguchi T, Fujii M, Takayama T. Mortality for gastric cancer in elderly patients. J Surg Oncol. 2003;84:132–136. doi: 10.1002/jso.10303. [DOI] [PubMed] [Google Scholar]
- 25.Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg. 1997;84:1567–1571. doi: 10.1111/j.1365-2168.1997.02842.x. [DOI] [PubMed] [Google Scholar]