Abstract
Cartilage oligomeric matrix protein/thrombospondin-5 (COMP/TSP5) is an abundant cartilage extracellular matrix (ECM) protein that interacts with major cartilage ECM components, including aggrecan and collagens. To test our hypothesis that COMP/TSP5 functions in the assembly of the ECM during cartilage morphogenesis, we have employed mesenchymal stem cell (MSC) chondrogenesis in vitro as a model to examine the effects of COMP over-expression on neo-cartilage formation. Human bone marrow-derived MSCs were transfected with either full-length COMP cDNA or control plasmid, followed by chondrogenic induction in three-dimensional pellet or alginate-hydrogel culture. MSC chondrogenesis and ECM production was estimated based on quantitation of sulfated glycosaminoglycan (sGAG) accumulation, immunohistochemistry of the presence and distribution of cartilage ECM proteins, and real-time RT-PCR analyis of mRNA expression of cartilage markers. Our results showed that COMP over-expression resulted in increased total sGAG content during the early phase of MSC chondrogenesis, and increased immuno-detectable levels of aggrecan and collagen type II in the ECM of COMP-transfected pellet and alginate cultures, indicating more abundant cartilaginous matrix. COMP transfection did not significantly increase the transcript levels of the early chondrogenic marker, Sox9, or aggrecan, suggesting that enhancement of MSC cartilage ECM was effected at post-transcriptional levels. These findings strongly suggest that COMP functions in mesenchymal chondrogenesis by enhancing cartilage ECM organization and assembly. The action of COMP is most likely mediated not via direct changes in cartilage matrix gene expression but via interactions of COMP with other cartilage ECM proteins, such as aggrecan and collagens, that result in enhanced assembly and retention.
Keywords: cartilage oligomeric matrix protein, mesenchymal stem cells, extracellular matrix, cartilage development
INTRODUCTION
Cartilage oligomeric matrix protein (COMP, also known as TSP5 in the literature) is an abundant component of cartilage extracellular matrix (ECM) (Murphy, et al, 1999; DiCesare, et al, 1996; DiCesare, et al, 2002). It is a pentameric ECM protein that can also be found in bone, tendon, ligament, synovium, and blood vessels (DiCesare, et al, 2000; DiCesare, et al 1997; DiCesare, et al,1994; Hebom, et al, 1992; Hecht, et al, 1998; Smith, et al, 1997). Its importance is suggested by its association with several pathological conditions. The functional importance of COMP to cartilage is underscored by its association with several joint diseases. Mutations in COMP cause heritable diseases of skeletal dysplasia including pseudoachondroplasia and certain forms of multiple epiphyseal dysplasia, conditions characterized by defective cartilage ECM, disrupted longitudinal growth and early onset of arthritis. COMP expression patterns and levels are altered in human arthritic conditions (Briggs, et al,1995; Hecht, et al, 1995; Cohn, et al, 1996; Briggs and Chapman, 2002). COMP degradation has been shown to correlate with cartilage destruction in arthritis of the knee and hip (DiCesare, et al, 1996; Neidhart, et al, 1997; Petersson, et al, 1998). Quantitative analysis shows a net loss of COMP from arthritic cartilage as compared to normal cartilage. Furthermore, COMP protein that remains in the arthritic cartilage shows increased degradation fragments as compared to the mostly intact COMP extracted from normal cartilage (DiCesare, et al, 1996). These studies suggest that a defect in COMP may have a direct impact on the ECM integrity and mechanical properties of the cartilage.
Although the association of COMP with the above pathologic conditions suggests its importance in cartilage, what role COMP plays in healthy cartilage, and during cartilage formation and cartilage ECM elaboration is not clear. COMP knockout mice do not show overt skeletal defect during development (Svensson, et al, 2002). However, COMP deficiency affects the animals’ susceptibility to arthritis development. When challenged with collagen type II induced arthritis, COMP knockout mice showed a significant early onset and increase in the severity in the chronic phase of arthritis, while not affecting collagen type II autoimmunity (Geng, et al, 2008). This finding indicates the importance of COMP in cartilage stability. To this end, COMP has been shown to be able to interact with collagen type II (Rosenberg, et al, 1998), and this interaction appears to catalyze and increase the rate of collagen fibril formation by promoting early association of collagens (Halasz, et al, 2007). In addition, we have previously shown that COMP can bind aggrecan, another major cartilage ECM component (Chen, et al, 2007). Due to the fact that COMP is a pentamer, these properties suggest that COMP has the potential to interact with multiple ECM proteins and participate in ECM organization. Furthermore, we have shown that COMP binds cells through cell surface integrin receptors (Chen, et al, 2005), and through this interaction, COMP can regulate intracellular signaling transduction pathways and possibly influence cellular phenotype development. These characteristics collectively suggest the potential importance of COMP to cartilage phenotype development, its matrix organization and load support function. Based on the above observation, we have hypothesized that COMP may function in cartilage development and assembly and organization of the cartilaginous ECM through interaction with the other major matrix components. To address this, we have used chondrogenically induced adult mesenchymal stem cells (MSCs) as an experimental model. MSCs are adult tissue-derived progenitor cells that have multi-lineage differentiation potentials (Chen and Tuan, 2008). Specifically, upon stimulation with transforming growth factor-β (TGF-β), high density 3-dimensional (3-D) cultures of MSC undergo distinct chondrogenic differentiation, expressing cartilage specific genes, including COMP, collage type II, and aggrecan (Li, et al, 2011), and elaborating a cartilage ECM (Yoo, et al, 1998). In this study, we have investigated the function of COMP during neo-cartilage ECM formation by MSCs in vitro. We have specifically analyzed the effects of COMP over-expression on MSC chondrogenesis and ECM formation and organization.
MATERIALS AND METHODS
Isolation and culture of MSC
Human MSCs were isolated from bone marrow obtained from patients undergoing total joint arthroplasty with the IRB approval of University of Washington (Seattle, WA) as previously described (Song and Tuan, 2004). Briefly, MSCs were isolated from bone marrow by means of direct substrate attachment, and were cultured in Dulbecco’s Modified Eagle’s Medium (DME) with high glucose containing 10% MSC-selected fetal bovine serum and antibiotics. MSCs were used between passages 2 and 3 at 70–80 % confluency.
MSC transfection and chondrogenesis
MSCs were transfected using the Amaxa Nucleofection method (Haleem-Smith, et al, 2005) (Lonza, Basel, Switzerland) either with an expression plasmid construct encoding the full length COMP cDNA (Chen, et al, 2000) or with a control plasmid. Following transfection, cells were allowed to recover in monolayer for two days. MSC chondrogenic differentiation was initiated by culturing the cells in high density 3-D pellet culture or in alginate culture supplemented with TGFβ3. Briefly, MSCs were trypsinized, resuspended as single cells, and 2.5 × 105 cells were pelleted at 300 × g for 7 min. Alginate cultures were formed essentially as described previously (Robbins and Goldring, 1998). Cells were suspended in 1.2% alginate at 4 × 106 cells/ml, and alginate was crosslinked with 102 mM CaCl2 for 10 min. Cell pellets and alginate beads were cultured in chemically defined chondrogenic medium: DME containing 1% ITS+ (BD Biosciences, Bedford, MA), 50 μg/ml ascorbate, 40 μg/ml proline, and 0.1 μM dexamethasone, containing recombinant human TGF-β 3 (R&D Systems, Minneapolis, MN) at 10 ng/ml. Culture medium was exchanged every 2–3 days until harvest.
Sulfated glycosaminoglycan (sGAG) Assay
Cartilage ECM formation was quantified by assaying total sGAG production by the cells. Pellets were digested with papain in GAG digestion buffer (0.1 M sodium acetate, pH 6.0, containing 10 mM cysteine, and 50 mM EDTA). An aliquot of the digest was assayed for sGAG content using the Blyscan kit (Accurate Chemical & Scientific Corp, Westbury, NY) according to the manufacturer’s instruction. Another aliquot of the digest was assayed for DNA content using the PicoGreen kit (Molecular Probes, Eugene, OR). For comparison, sGAG content was normalized by DNA content.
Immunoblotting
To assess the efficiency of COMP transfection, culture media of pellet cultures were collected, and analyzed by immunoblotting using a rabbit polyclonal antibody F8, directed against COMP (Chen, 2000; Hecht, et al, 1998). Samples were electrophoretically separated on an 8% SDS polyacrylamide gel. Recombinant human COMP was used as a positive control (Chen and Lawler, 2001). Proteins were electro- transferred to a nitrocellulose membrane, and nonspecific binding was blocked with 5% powdered milk in Tris-buffered saline. F8 antibody was used at a dilution of 1:1000. Following incubation with horseradish peroxidase conjugated goat anti-rabbit secondary antibody (Pierce Biotechnology Inc, Rockford, IL), and immunoreactive proteins were detected by enhanced chemiluminescence (Pierce).
Histology and immunohistochemistry
Pellets and alginate cultures were fixed with 4% paraformaldehyde, and alginate beads were further crosslinked in 100 mM BaCl2. After embedding in paraffin, 5 μm sections were obtained, and stained with Alcian Blue (Rowley Biochemical Inst, Danvers, MA) for matrix sGAG. The presence and distribution of cartilage specific ECM proteins was examined by immunohistochemistry using the following antibodies: aggrecan, 12/21/I-C-6 (DSHB-Developmental Studies Hybridoma Bank, Iowa City, IA); type II collagen, II-II-6B3 (DSHB); and COMP, F8 (Chen and Lawler, 2001). After secondary labeling with horseradish peroxidase conjugated secondary antibodies, the Histostain kit (Zymed. San Francisco, CA) was applied for detection with diaminobenzidine as the chromogen, in accordance with the manufacturer’s instructions. After staining, the slides were mounted in Clarion mounting medium (Biomeda, Foster City, CA) for observation under the microscope.
Quantitative real time RT-PCR (QPCR)
Pellet cultures were harvested in Trizol (Invitrogen, Carlsbad, CA). Total RNA was isolated, and cleaned up using RNeasy (Qiagen, Valencia, CA). The amount of RNA was determined spectrophotometrically as A260. 1 μg aliquot of total RNA was reverse transcribed using Omniscript reverse transcriptase (Qiagen) and oligo(dT) primers (Invitrogen). CybrGreen based QPCR was performed using gene-specific primers, including (1) COMP, 5’-AACAGTGCCCAGGAGGAC-3’ (sense) and 5’-TTGTCTACCACCTTGTCTGC-3’ (anti-sense); (2) aggrecan, 5’-TCCCCACGGTCTCTCTTGTAG-3’ (sense) and 5’-GCCCACTTAGGTCCAGAAATCC-3’ (anti-sense); (3) Sox9, 5’-GCAGGCGGAGGCAGAGGAG-3’ (sense) and 5’-GGAGGAGGAGTGTGGCGAGTC-3’ (anti-sense); and (4) HPRT, 5’-CGAGATGTGATGAAGGAGATGG-3’ (sense) and 5’-GCAGGTCAGCAAAGAATTTATAGC-3’ (anti-sense). The house keeping gene, HPRT, was used as a control for RNA loading. Relative gene expression was determined using the standard curve method.
RESULTS
COMP cDNA transfection increases COMP protein level
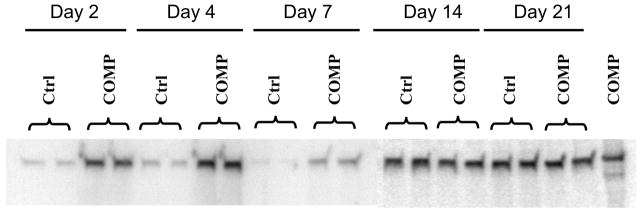
MSCs were transfected with full length COMP cDNA, and maintained as pellet cultures in chondrogenic medium. The culture media collected from COMP transfected and control vector transfected cultures at various time points were immunoblotted for secreted COMP protein (Figure 1). Higher levels of COMP were detected in COMP-transfected cultures compared to the control transfected cultures during early time points after transfection, indicating that COMP cDNA transfection of MSCs effectively increased their COMP protein production. However, the increase in COMP production was not obvious at later time points of 14 and 21 days after transfection (Figure 1).
Figure 1. COMP production by MSCs cultures during chondrogenic differentiation.
MSCs were transfected with full length COMP cDNA (COMP) or control vector (Ctrl), and induced to undergo chondrogenic differentiation in pellet cultures. Culture media were collected at the days indicated, and COMP protein was detected by immunoblotting using F8 polyclonal antibodies against COMP. Duplicate cultures of each are shown. Immunoblot of purified COMP is shown in the rightmost lane. Increased COMP production was seen in COMP-transfected cells at early culture times.
COMP overexpression increases sGAG content
High density cultures of MSCs in TGFβ3 supplemented chondrogenic medium underwent chondrogenesis and produced cartilage like ECM including the accumulation of cartilage proteoglycans, COMP, and collagen type II (Barry, et al, 2001; Sekiya, et al, 2002). Biochemical analysis showed that sGAG content increased with time in both control and COMP transfected pellets. At weeks 1 and 2, sGAG contents were significantly higher in COMP transfected pellets, compared to the control transfected pellets (Figure 2), suggesting higher levels of sulfated proteoglycans in the ECM upon overexpression of COMP. The difference was no longer significant after three weeks of culture (data not shown).
Figure 2. sGAG production by MSC pellet cultures during chondrogenesis.
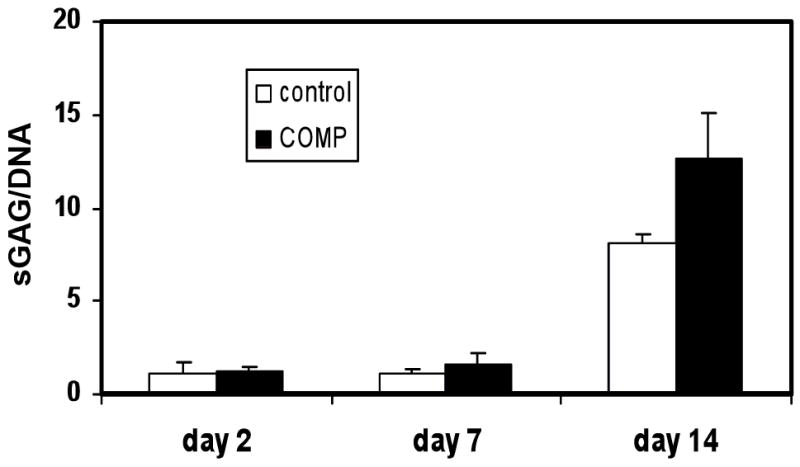
Pellet cultures of MSCs, transfected with full length COMP cDNA (COMP) or control vector (Control), were induced to undergo chondrogenic differentiation, harvested at the indicated time points, and analyzed for sGAG content using the Blyscan kit. DNA contents of the pellets were determined using PicoGreen assay and used for normalization of sGAG contents. The results showed that COMP over-expression resulted in higher sGAG production during the first 2 weeks of MSC chondrogenesis. Values are mean ± SD (n = 5).
Distribution of cartilage ECM components in pellet cultures
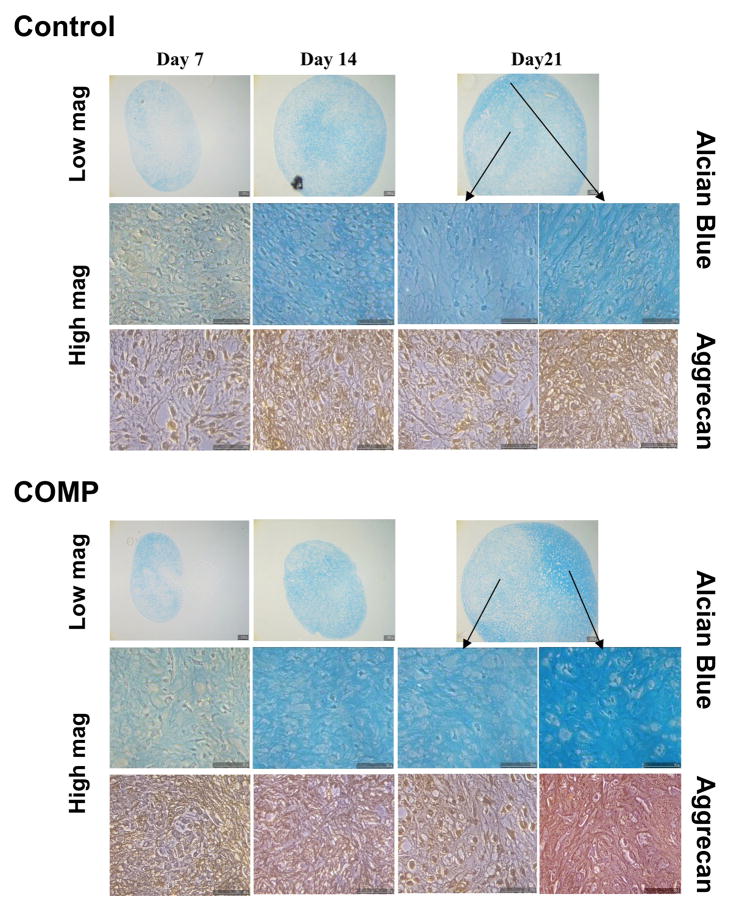
The increased sGAG contents in COMP transfected pellets were confirmed histologically by alcian blue staining, which showed culture time-dependent increase in staining intensity in both control and COMP transfected samples. Figure 3 depicts both low magnification and high magnification views to illustrate the overall and regional staining profiles, respectively. Consistent with the quantitative sGAG data, COMP transfected cultures showed overall more intense staining throughout the cell pellet, indicating higher total sGAG content (Figure 3, Top). Higher magnification revealed the more intense staining in the ECM of COMP transfected samples. To confirm the presence of sulfated cartilage specific proteoglycans, aggrecan immunohistochemistry was carried out (Figure 3, Bottom). In agreement with the alcian blue observation, aggrecan immunostaining in COMP transfected samples also appeared to be stronger in intensity, and more extensive and evenly distributed throughout the ECM. Taken together, these findings indicate that a more cartilaginous and organized ECM was produced in the COMP transfected cultures.
Figure 3. Histological analysis of MSC pellet cultures undergoing chondrogenesis: Alician blue for sGAG and immunohistochemistry for aggrecan.
MSCs were transfected with full length COMP cDNA (COMP) or control vector (Control), and induced to undergo chondrogenic differentiation in pellet cultures. Pellets were harvested at the indicated time points and fixed and processed for histology. Both low and high magnifications of the staining are included, showing more prominent and distinct staining for sGAG (Alcian blue) and aggrecan in the overall pellet as well as in pericellular matrix of the newly formed cartilage of COMP-transfected cultures from culture day 14 on.
COMP overexpression increases cartilage matrix protein levels in alginate cultures
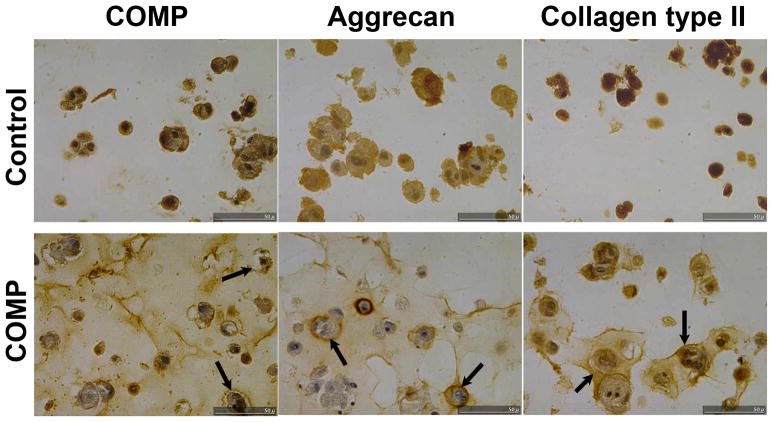
Three-dimensional culture of MSC in the hydrogel, alginate, represents another system for efficient induction of chondrogenesis in vitro (Yang, et al, 2004; Majumdar, et al, 2000). Unlike pellet cultures, cell rounding, instead of direct cell-cell contact and cell condensation, is postulated to be responsible for the promotion of chondrogenesis in MSC suspension cultures in alginate. MSCs underogoing chondrogenesis in alginate have been shown to display phenotypic characteristics slightly different from those from pellet culture, and have been suggested to more closely resemble articular cartilage (Yang, et al, 2004). We therefore tested whether COMP transfection could also improve the outcome of MSC chondrogenesis in alginate. Figure 4 shows representative staining of cartilage matrix proteins, including COMP, aggrecan, and collagen type II, in alginate MSC cultures at 21 days after transfection. As expected, COMP immunostaining in the ECM was higher in COMP transfected cultures. Compared to control transfected samples, COMP transfected cultures exhibited considerably stronger ECM staining for both aggrecan and collagen type II, particularly in pericellular areas. Specifically, both the intensity and the area of positive staining were increased in the ECM of the COMP transfected cells as compared to the control.
Figure 4. Immunohistolochemical staining of COMP, aggrecan, and collagen type II of MSCs during chondrogenesis in alginate hydrogel cultures.
MSCs were transfected with full length COMP cDNA (COMP) or control vector (Control), and induced to undergo chondrogenic differentiation in alginate cultures which were harvested at day 21, fixed and processed for immunohistochemistry to localize COMP, aggrecan and collagen type II. In addition to the expected higher staining intensity of COMP, the COMP-transfected ultures displayed substantially more intense and distributed staining for both aggrecan and collagen type II, localized to the immediate pericellular matrix (arrows) within the alginate hydrogel.
Effect of COMP over expression on cartilage marker gene expression
We have previously shown that COMP can bind to chondrocyte cell surface receptors α5β1 and αvβ3 (Chen, et al, 2005). Through this interaction, it is plausible that COMP can influence intracellular signaling and cellular phenotype. QPCR was next performed to investigate whether the chondrogenesis-enhancing effect of COMP over-expression was a result of increased mRNA expression of chondrocyte-specific genes. The results showed that while transfection of COMP caused the expected increase in COMP mRNA levels, there was no significant increase in the transcript levels of the early chondrogenic marker, Sox9, or of aggrecan, compared to control (Figure 4). This finding suggests that a direct transcriptional effect of COMP over-expression to enhance MSC chondrogenesis was unlikely.
DISCUSSION
In this study, we have investigated the effect of COMP over-expression on human bone marrow-derived MSCs undergoing chondrogenic differentiation. Our results showed that increased expression of COMP led to increased levels of the major ECM components, including aggrecan and collagen type II. This increase was shown quantitatively by sGAG assay during the early time points, and qualitatively by immunohistochemical staining of the major cartilage markers of aggrecan and collagen type II, with higher levels in the COMP transfected pellet and alginate cultures, concomitant with the appearance of more abundant matrix. This increase in cartilage matrix level could be a result of enhanced chondrogenic differentiation of MSCs. To test this possibility, we analyzed the mRNA expression of early chondrogenic marker genes by QPCR, including Sox 9, the master chondrogenic transcription factor. Interestingly, over-expression of COMP did not result in increased mRNA levels of Sox9 or aggrecan. A recent report by Roman-Blas, et al (2010) employed chicken limb bud micromass cultures transduced to express exogenous wild-type or mutant COMP constructs. They also observed that over-expression of wild-type COMP did not affect expression levels of the chondrocyte-associated genes, collagen type II and matrillin-3, but increased sulfated proteoglycan production. Taken together, these results thus suggest that the observed increased in cartilage ECM accumulation is mostly due to enhanced matrix organization instead of enhanced chondrogenic differentiation of MSCs at the gene expression level.
Our finding that higher levels of COMP correlate with higher levels of other major cartilage matrix proteins is in agreement with previous published reports suggesting a potential role of COMP in cartilage load support. These studies showed that the expression of COMP seems to correlate with the load-bearing function of tissues. During mouse limb development, COMP expression coincides with the elaboration of a weight-bearing chondrocyte matrix (Murphy, et al, 1999). In addition, high COMP expression has been shown to be localized to anatomic sites that sustain heavy loads and correlate with the elaboration of a weight-bearing chondrocyte matrix. For example, articular joint cartilage experiences higher load than cartilage from other organs. Correspondingly, articular cartilage expresses greater levels of COMP (5 mg/g wet weight) than rib and tracheal cartilage (0.26, and 0.04 mg/g wet weight, respectively) (Hauser, et al, 1995). In the same animal, tendons with the highest loading contain the greatest levels of COMP (Smith, et al, 1997). The above observations suggest that in vivo, COMP is synthesized in response to load, and its presence may be necessary for cartilage and tendon to carry out its load support function.
Articular cartilage in normal joints can resist, withstand, and transmit large loads (Mankin, et al, 1994; Ratcliffe and Mow, 1996; Mow and Ratcliffe, 1997). This unique function depends on the structural composition and organization of its ECM. The ability of articular cartilage to resist compression is primarily due to the presence of proteoglycan aggregates. High density, fixed negative charges of the sGAG chains draw water into the tissue (Maroudas, 1979; Buschmann and Grodzinsky, 1995; Lai, et al, 1997; Linn and Sokoloff, 1965; Hardingham, et al, 1992), resulting in a large osmotic pressure (Hardingham, et al, 1992). The fluid phase of cartilage is important for its function, being able to support >90% of applied stress, shielding the matrix from excess deformation (Soltz and Ateshian, 1998). It has been shown that sGAG loss with change in water content is an early sign for cartilage degeneration and deceased function. Cartilage tensile stiffness and strength, on the other hand, is determined primarily by the collagen network (Kempson, et al, 1970). In addition, collagen can also contribute to the compressive behavior of cartilage (Williamson, et al, 2001). Thus, the biomechanical properties of articular cartilage are highly dependent on the integrity of the ECM network and on the maintenance of high proteoglycan content within the matrix. Our findings here show that higher contents of COMP result in higher levels of sGAG, aggrecan and collagen type II, and better matrix organization in newly formed cartilage derived from chondrogenic differentiation of adult human MSCs. Taken together with previous published results on the interaction of COMP with both collagens and aggrecan, our results are consistent with the proposed function of COMP in cartilage matrix organization and load support via its interaction with the two other major components of aggrecan and collagen.
Figure 5. Expression of early cartilage marker genes in MSC pellet cultures undergoing chondrogenesis.
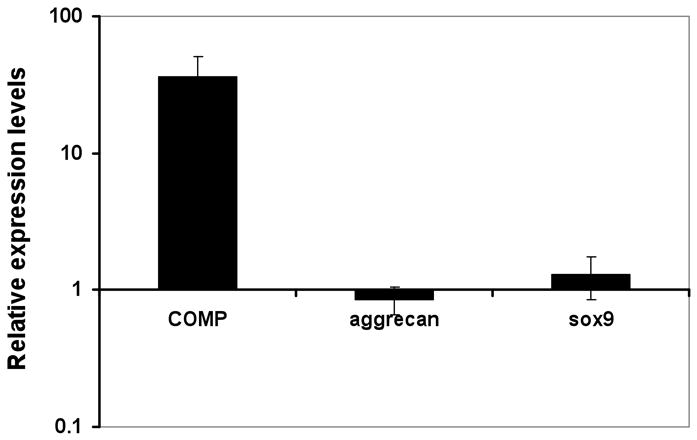
MSCs were transfected with full length COMP cDNA or control vector, induced to undergo chondrogenic differentiation in pellet cultures, and harvested at day 7 for RNA isolation and QPCR. mRNA expression levels of COMP, aggrecan, and Sox9 were normalized to that of the housekeeping gene, HPRT. Relative mRNA expression level of each gene was expressed as ratios of normalized gene expression of COMP transfected pellets over that of control transfected pellets. Except for the expected, more than 50-fold increase in COMP RNA levels in the COMP-transfected cultures, no detectable difference was seen for either Sox 9 or aggrecan. Values are mean ± SD (n = 5).
Acknowledgments
This work was funded in part by the Intramural Research Program of the National Institutes of Health (Z01 AR41131), the Howard Hughes Medical Institute-National Institutes of Health Research Scholars Program, and the Commonwealth of Pennsylvania Department of Health.
References
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268(2):189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Briggs MD, Chapman KL. Pseudoachondroplasia and multiple epiphyseal dysplasia: mutation review, molecular interactions, and genotype to phenotype correlations. Hum Mutat. 2002;19(5):465–78. doi: 10.1002/humu.10066. [DOI] [PubMed] [Google Scholar]
- Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10(3):330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:170–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- Chen H, Deere M, Hecht JT, Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein and a mutation in its type 3 repeats causes conformational changes. J Biol Chem. 2000;275(34):26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- Chen FH, Herndon ME, Patel N, hecht JT, Tuan Rs, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282(34):24591–24598. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J Biol Chem. 2005;280(38):32655–32661. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10(5):223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein, and a mutation in its type 3 repeats causes conformational changes. J Biol Chem. 2001;275(34):26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- Cohn DH, Briggs MD, King LML, Rimoin DL, Wilcox WR, Lachman RS, Knowlton RG. Mutations in the cartilage oligomeric matrix protein (COMP) gene in pseudoachondroplasia and multiple epiphyseal dysplasia. Ann NY Acad Sci. 1996;785:188–194. doi: 10.1111/j.1749-6632.1996.tb56258.x. [DOI] [PubMed] [Google Scholar]
- Di Cesare PE, Carlson CS, Stollerman ES, Hauser N, Tulli H, Paulsson M. Increased degradation and altered tissue distribution of cartilage oligomeric matrix protein in human rheumatoid and osteoarthritic cartilage. J Orthop Res. 1996;14:946–955. doi: 10.1002/jor.1100140615. [DOI] [PubMed] [Google Scholar]
- Di Cesare PE, Carlson CS, Stollerman ES, Chen FS, Leslie M, Perris R. Expression of cartilage oligomeric matrix protein by human synovium. FEBS Lett. 1997;412(1):249–252. doi: 10.1016/s0014-5793(97)00789-8. [DOI] [PubMed] [Google Scholar]
- Di Cesare P, Chen FS, Moergelin M, Carlson CS, Leslie MLP, Perris R, Fang C. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002;21(5):461–470. doi: 10.1016/s0945-053x(02)00015-x. [DOI] [PubMed] [Google Scholar]
- Di Cesare PE, Fang C, Leslie lMP, Tulli H, Perris R, Carlson CS. Expression of cartilage oligomeric matrix protein (COMP) by embryonic and adult osteoblasts. J Orthop Res. 2000;18(5):713–720. doi: 10.1002/jor.1100180506. [DOI] [PubMed] [Google Scholar]
- Di Cesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354(2):237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- Geng H, Carlsen S, Nandakumar KS, Holmdahl R, Aspberg A, Oldberg A, Mattsson R. Cartilage oligomeric matrix protein deficiency promotes early onset and the chronic development of collagen-induced arthritis. Arthritis Res Ther. 2008;10(6):R134. doi: 10.1186/ar2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282(43):31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- Haleem-Smith H, Derfoul A, Okafor C, Tuli R, Olsen D, Hall DJ, Tuan RS. Optimization of high-efficiency transfection of adult human mesenchymal stem cells in vitro. Mol Biotechnol. 2005;30(1):9–20. doi: 10.1385/MB:30:1:009. [DOI] [PubMed] [Google Scholar]
- Hardingham TE, Fosang AJ, Dudhia J. Aggrecan, the chondroitin sulfate/keratin sulfate proteoclycan from cartilage. In: Kuettner K, editor. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992. pp. 5–20. [Google Scholar]
- Hauser N. Distribution of CMP amd COMP in human cartilage. Acta Orthop Scand. 1995;266 (Suppl 266):71–79. [Google Scholar]
- Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17(4):269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Montufar-Solis D, Decker G, Lawler J, Daniels K, Duke PJ. Characterization of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol. 1998;17:625–633. doi: 10.1016/s0945-053x(98)90113-5. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10(3):325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- Hedbom E, Antonsson P, Hierpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heingard D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267(9):6132–6136. [PubMed] [Google Scholar]
- Kempson GE, Muir H, Swanson SA, Freeman MA. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113(3):245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- Li H, Haudenschild DR, Posey KL, Hecht JT, DiCesare PE, Yik JH. Comparative analysis with collagen type II distinguishes cartilage oligomeric protein as a primary TGFβ-responsive gene. Osteoarthritis Cartilage. 2011;19(10):1246–1253. doi: 10.1016/j.joca.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn FC, Sokoloff L. Movement and composition of interstitial fluid of cartilage. Arthritis Rheum. 1965;8:481–494. doi: 10.1002/art.1780080402. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Banks V, Peluso DP, Morris EA. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000;185(1):98–106. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mankin HJ. Structure and function of articular cartilage. In: Simon SR, editor. Orthopaedic Basic Science. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1994. pp. 1–44. [Google Scholar]
- Maroudas A. Physicochemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Kent, England: Pitman Medical Publishing; 1979. pp. 215–290. [Google Scholar]
- Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow, Hayes, editors. Basic Orthopaedic Biomechanics. Philadelphia, PA: Lippincott–Raven; 1997. pp. 113–178. [Google Scholar]
- Murphy JM, Heinegard R, McIntosh A, Sterchi D, Barry FP. Distribution of cartilage molecules in the developing mouse joint. Matrix Biol. 1999;18(5):487–497. doi: 10.1016/s0945-053x(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36:1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- Petersson IF, Boegard T, Svensson B, Heinegard D, Saxne T. Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol. 1998;37:46–50. doi: 10.1093/rheumatology/37.1.46. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Mow VC. The structure and function of articular cartilage. In: Competer WD, editor. Structure and Function of Connective Tissues. Amsterdam, The Netherlands: Harwood Academic Press; 1996. pp. 234–302. [Google Scholar]
- Robbins JR, Goldring MB. Methods for preparation of immortalized human chondrocyte cell lines. In: Morgan JR, Yarmush MR, editors. Methods in Molecular Medicine: Tissue Engineering Methods and Protocols. Humana Press, Inc; Totowa, NJ: 1998. pp. 173–194. [DOI] [PubMed] [Google Scholar]
- Roman-Blas J, Dion AS, Seghatoleslami MR, Giunta K, Oca P, Jimenez SA, Williams CS. MED and PSACH COMP mutations affect chondrogenesis in chicken limb bud micromass cultures. J Cell Physiol. 2010;224(3):817–826. doi: 10.1002/jcp.22185. [DOI] [PubMed] [Google Scholar]
- Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99(7):4397– 4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RK, Zunino L, Webbon PM, Heinegard D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997;16:255–271. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18(9):980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Heinegard D, Hunziker EB, Reinholt FP, Fassler R, Oldberg A. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol. 2002;22(12):4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19(6):1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Yang IH, Kim SH, Kim YH, Sun HJ, Kim SJ, Lee JW. Comparison of phenotypic characterization between “alginate bead” and “pellet” culture systems as chondrogenic differentiation models for human mesenchymal stem cells. Yonsei Med J. 2004;45(5):891–900. doi: 10.3349/ymj.2004.45.5.891. [DOI] [PubMed] [Google Scholar]
- Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Golberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80(12):1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]