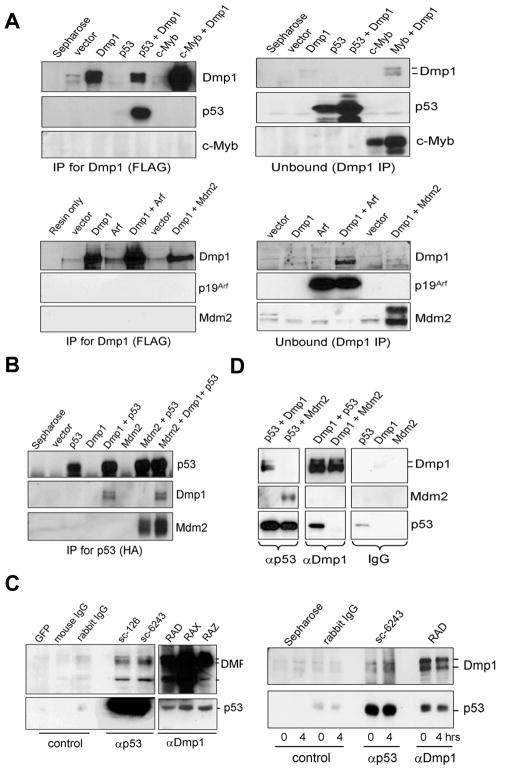
Fig. 1. Dmp1 co-immunoprecipitates with p53.
(A), (B) NIH 3T3 cells were transfected with cDNA for FLAG-Dmp1, HA-p53, c-Myb, p19Arf, and/or Mdm2 expression vectors. The cleared lysates were immunoprecipitated with antibodies to (A) Dmp1 (FLAG) or (B) p53 (HA), and analyzed by Western blotting.
(C, left panels) Endogenous Dmp1-p53 interaction in U2OS cells treated with etoposide. Etoposide/MG-132 treated lysates were immunoprecipitated with control GFP antibody (sc-9996), control IgGs or antibodies to p53 (sc-126, sc-6243) or Dmp1 (RAD, RAX, RAZ), followed by blotting with Dmp1 or p53 antibodies.
(C, right panels) Endogenous Dmp1-p53 interaction in the mouse thymus injected with etoposide. Lysates were immunoprecipitated with control immunoglobulins, p53 antibody (sc-6243), or Dmp1 antibody (RAD) and analyzed by Western blotting.
(D) Recombinant FLAG-Dmp1, HA-p53, and FLAG-Mdm2 proteins were purified from baculovirus-infected Sf9 cells. In vitro binding assays were conducted by using 4 μg of proteins followed by immunoprecipitation with p53 (sc-6243), Dmp1 (RAX), or control IgGs, and analyzed by Western blottings.