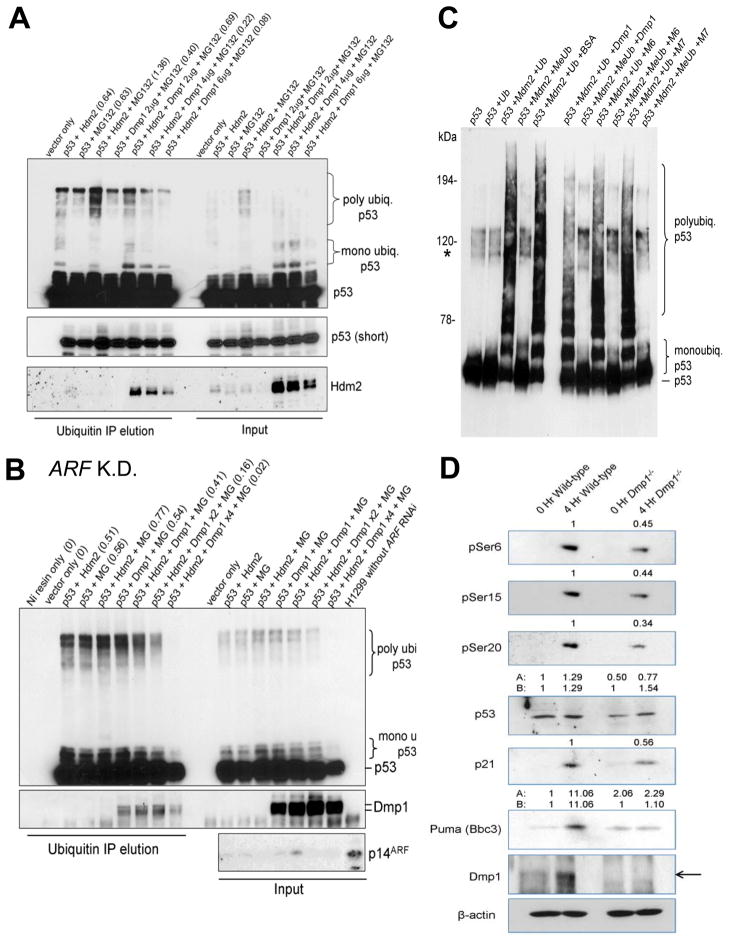
Fig. 3. Dmp1 modulates p53 ubiquitination in vivo and in vitro.
(A) Dmp1 modulates p53 ubiquitination. H1299 cells were transfected with His-ubiquitin, p53, Hdm2, and increasing amounts of Dmp1. Cells were treated with the proteasome inhibitor MG-132 and ubiquitinated p53 was isolated with Ni-NTA resin and analyzed by Western blotting with the indicated antibodies. The numbers in the parentheses show densitometric values of polyubiquitinated p53.
(B) In cell p53 ubiquitination assay using H1299 cells transfected with p14ARF shRNA. Polyubiquitination of p53 by Hdm2 was significantly inhibited by Dmp1 (47% by Dmp1 x1, 79% by Dmp1 x2, and 98% by 4x Dmp1) expression in H1299 cells with 90% downregulation of p14ARF by specific shRNA, indicating that the inhibitory effect of Dmp1 on p53 ubiquitination was independent of ARF. K.D.: knockdown; MG: MG132.
(C) Dmp1 blocks p53 ubiquitination by Mdm2 in vitro. In vitro ubiquitination assays were performed in the presence of purified p53, Mdm2 (E3), E2 (ubch5a), E1, and ATP using either ubiquitin (poly- and mono-ubiquitination) or methyl-ubiquitin (mono-ubiquitination only). Dmp1 M6 and M7 mutants that do not interact with p53 did not block poly- or mono-ubiquitination. Asterisk indicates endogenous Sf9 ubiquitination of p53.
(D) Both total and phosphorylated p53 levels are reduced in Dmp1−/− thymus compared to wild-type. Total protein was isolated from the thymus of Dmp1-null and wild-type mice after intravenous injection with doxorubicin for 4 hours and resolved by SDS-PAGE. The numbers indicate relative expression levels of each protein determined by densitometric analyses of Western blotting data.