Abstract
Objectives
To retrospectively analyze all patients who were diagnosed with Osteomyelitis of jaws in our unit.
Study Design
31 patients with Osteomyelitis of jaws were analyzed retrospectively from 2002 to 2008 at the Department of Oral & Maxillofacial Surgery, S.D.M College of dental sciences and hospital, Dharwad, India. Parameters considered were age, gender, jaws involved, clinical features, surgical management & complications.
Results
Of the 31 patients, maxilla was involved in 16 patients and mandible was involved in the remaining 15. 11 out of the 16 patients with maxillary osteomyelitis were immuno-compromised. The predominant etiology noted was odontogenic infection. With the treatment protocol we have adopted, all our patients showed satisfactory resolution of the condition by 6 weeks.
Conclusion
Incidence of maxillary osteomyelitis & their association with diabetes mellitus was higher in our series compared to others. The cause for this high incidence was analyzed in our study. Based on our results we conclude that a conservative surgical method with an attempt to preserve vital bone and an appropriate antibiotic therapy with the correction of the underlying medical problems is adequate to treat Osteomyelitis of jaws.
Keywords: Osteomyelitis, Maxilla, Mandible, Diabetes mellitus
Introduction
Osteomyelitis is considered as “an inflammatory condition of the bone that usually begins as an infection of the medullary cavity, rapidly involves the haversian systems and quickly extends to the periosteum of the area” [1]. In the Pre-Antibiotic era, Osteomyelitis of the jaws was a more frequently encountered, many a times, a fatal infection in the maxillofacial region. However the discovery of antibiotics and antimicrobial chemotherapeutic agents, better surgical treatment options and use of Hyperbaric oxygen therapy has greatly influenced the incidence and prognosis of this disease.
In recent times there appears to be a change in the clinical presentation of this pathology. This can mainly be attributed to the increasing incidence of systemic diseases that compromise the host immune factors such as diabetes mellitus, HIV infections, use of immune suppressing drugs, malnutrition etc.
It is a well-established fact that osteomyelitis affects maxilla less frequently than mandible. This is due to the significant collateral blood flow in the midface & the porous nature of membranous maxillary bone [2]. Contrary to this,an unusually high incidence of maxillary osteomyelitis was seen at our unit. Hence a retrospective study of all the cases of osteomyelitis of the jaw bones that reported to our unit between 2002 and 2008(6 year period) was conducted.
The aim of this study is to:
Evaluate all the cases of osteomyelitis of jaws that were treated at our unit for a period of 6 years (between 2002 and 2008) with respect to Age, Sex, site, etiology, presenting clinical signs & symptoms, contributing medical conditions, various micro-organisms involved and their follow up.
To evaluate the possible cause for the increasing trend in the incidence of maxillary osteomyelitis.
To evaluate the efficacy of our treatment protocol in treating osteomyelitis.
Materials & Methods
A total of 247 case records of patients with oro-facial infections that were treated in our unit during the 6 year period of 2002–2008 were analyzed. Out of these, 31 patients had osteomyelitis of the jaws. In these patients, standard data collection included age, sex, the jaw involved, clinical features,culture sensitivity reports, treatment given, post treatment follow up and complications. In all the patients, the diagnosis was based on clinical signs & symptoms, radiographic findings and histo-pathologic report.
Treatment Protocol Followed
All the patients diagnosed with osteomyelitis of the jaws were treated with a standardized protocol that is followed in our unit. The protocol is as follows:
Evaluation & correction of the underlying systemic disorder.
Pus if present for Gram staining, culture sensitivity.
Broad spectrum empirical antibiotic therapy.
Imaging and biopsy for histopathological reporting and culture (suspected fungal cases)
Definitive surgical treatment.
Administration of culture guided antibiotics if empirical antibiotics were clinically found ineffective.
Motivating the patient to stop deleterious habits.
Results
Out of 31 patients, 21 patients were male & 10 patients were female with the age ranging from 10 years to 65 years with a mean age of 44.
In our series of patients we encountered a higher incidence of osteomyelitis in maxilla compared to mandible i.e. 16 out of 31 patients (51.7%).
The various etiological causes, habits, presenting symptoms, medical status and culture sensitivity results are summarized in Table 1.
Table 1.
Clinical findings
| Patients | Jaw | Complaint | Etiology | Medical status | Microorganisms cultured |
|---|---|---|---|---|---|
| Patient 1 | Mandible | Pain in lower jaw, paresthesia, halitosis, mobile teeth | Odontogenic | None | No growth |
| Patient 2 | Mandible | Pain, swelling, pus discharge, paresthesia, halitosis, mobile teeth | Odontogenic | Diabetic & anaemic | No growth |
| Patient 3 | Mandible | Pain, swelling, fever, paresthesia | Odontogenic | None | No growth |
| Patient 4 | Maxilla | Fistula in roof of mouth since 1.5 years | Unknown | None | No growth |
| Patient 5 | Maxilla | Pus discharge, bone exposure of post maxilla | Maxillary sinusitis | Diabetic & hypertension | Mucormycosis growth |
| Patient 6 | Mandible | Pain, pus discharge in chin region | Odontogenic | None | Staph Aureus, Klebsella pneumonia |
| Patient 7 | Mandible | Pus discharge in lower back jaw region | Odontogenic | Dust allergy | No growth |
| Patient 8 | Mandible | Pain, swelling in R lower back jaw region since 1 month | Odontogenic | None | No growth |
| Patient 9 | Mandible | Pain in lower jaw, 3years | Odontogenic | None | No growth |
| Patient 10 | Maxilla | Pain in left middle face, 1 month. Pus discharge & halitosis | Odontogenic | None | No growth |
| Patient 11 | Maxilla | Pain in upper ant arch, 3 months, pus discharge | Odontogenic | Diabetic | Diphtheroids growth |
| Patient 12 | Maxilla | Swelling & pus discharge in upper left maxilla since 2 months | Odontogenic | Diabetic | (extended spectrum beta lactamase producing bacteria) ESBL |
| Patient 13 | Mandible | Pain,swelling,pus discharge from R & L side of lower jaw since 6 months, fever | Odontogenic | None | No growth |
| Patient 14 | Maxilla | Pain in roof of mouth, nasal regurgitation since 2 months | Right Max sinusitis | None | No growth |
| Patient 15 | Mandible | Pus discharge from Right cheek region since 20 days, fever | Odontogenic | Diabetic | No growth |
| Patient 16 | Maxilla | c/o mobility of maxillary anterior segment & oro nasal communication | Maxillary sinusitis | Diabetic | Mucormycosis |
| Patient 17 | Maxilla | c/o loss of sensation in the mid face & speech problem since 1 yr | Maxillary sinusitis | Diabetic & hypertension | Mucormycosis |
| Patient 18 | Maxilla | c/o solitary swelling in the roof of mouth,pain in upper jaw, nasal block since 1.5yrs | Odontogenic | Diabetic & hypertension | No growth |
| Patient 19 | Maxilla | c/o mobile ant maxilla, halitosis | Maxillary sinusitis | Diabetic & hypertension | Aspergillosis |
| Patient 20 | Maxilla | Pain & swelling in anterior maxilla, halitosis | Odontogenic | Diabetic & hypertension | G-ve bacilli + pus cells |
| Patient 21 | Mandible | c/o pain in the left side of lower jaw since 1 month | Odontogenic | Hypertension | No growth |
| Patient 22 | Mandible | c/o pain in Right lower back jaw region-1 month | Odontogenic | Lactating mother | No growth |
| Patient 23 | Mandible | c/o pain, swelling in R lower region of face since 5 months | Odontogenic | Hypertension | No growth |
| Patient 24 | Mandible | c/o pain & discharge in the R side of the lower jaw since 2 months | Following radiotherapy | None | Pseudomonas aeroginosa, staph aureus |
| Patient 25 | Mandible | c/o tooth ache since 45 days, swelling since 20 days, pus discharge-15 days from L side of mandible | Odontogenic | None | No growth |
| Patient 26 | Maxilla | Pain in left middle face,1 month. Pus discharge & halitosis | Odontogenic | None | No growth |
| Patient 27 | Maxilla | c/o nasal discharge & foul smelling since 1.5 years | Max sinusitis | Diabetic | Candida spp, E coli, enterococcus spp |
| Patient 28 | Maxilla | c/o solitary swelling in the roof of mouth,pain in upper jaw, nasal block since 1.5yrs | Odontogenic | Diabetic & hypertension | No growth |
| Patient 29 | Mandible | Pus discharge from Right cheek region, since 20 days | Odontogenic | Diabetic & anaemic | No growth |
| Patient 30 | Maxilla | Pain in upper ant maxilla, since 3 months pus discharge | Odontogenic | Diabetic | Enterococcus spp |
| Patient 31 | Maxilla | c/o mobility of upper ant teeth & pus discharge from right nostril & mouth since 8 months | Odontogenic | Hypertension | Enterococcus spp |
The most common etiology was odontogenic infections.
15 out of 31 patients had associated deleterious habits like tobacco chewing, smoking, alcoholism.
22 out of 31 patients, had an underlying systemic problems (70.9%).
An interesting finding is that, 11 out of 16 patients with maxillary osteomyelitis (almost 70%) reported with history of diabetes mellitus on admission as compared to 3 out of 15 patients with mandibular osteomyelitis(20%). The diabetic status of all the 14 patients diagnosed with osteomyelitis of jaws is listed in Table 2.
Table 2.
Diabetic status of the patients
| Patient | Duration of diabetes mellitus | Status on admission | Jaw |
|---|---|---|---|
| Patient 2 | 15 months | Poor control | Mandible |
| Patient 5 | 1.5 months | Good control | Maxilla |
| Patient 11 | 5 years | Poor control | Maxilla |
| Patient 12 | 15 days | Moderate control | Maxilla |
| Patient 15 | First time detected | Moderate control | Mandible |
| Patient 16 | 15 years | Moderate control | Maxilla |
| Patient 17 | 5 years | Good control | Maxilla |
| Patient 18 | 14 years | Moderate control | Maxilla |
| Patient 19 | 10 years | Moderate control | Maxilla |
| Patient 20 | 2 years | Poor control | Maxilla |
| Patient 27 | 4 years | Moderate control | Maxilla |
| Patient 28 | 14 years | Moderate control | Maxilla |
| Patient 29 | 8 years | Moderate control | Mandible |
| Patient 30 | 5 years | Poor control | Maxilla |
Glycosylated haemoglobin (Hb1ac values): Good control 6.4 to 7.5, Moderate control 7.6 to 9.0, Poor control above 9.0
The management of the patients principally consisted of controlling the underlying systemic illness followed by the definitive treatment of osteomyelitis. All the patients were started pre-operatively with a course of Empirical antibiotic therapy summarized in Table 3.
Table 3.
Treatment and post-operative review
| Patients | Antibiotics used | Definitive treatment performed | Duration of intra-venous post-op antibiotics | Hospital stay | Post-op complications |
|---|---|---|---|---|---|
| Patient 1 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction, decortications, Recon plate | 11 days | 14 days | Paresthesia |
| Patient 2 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction, saucerisation, decortications, ORIF with 2.5 mm SS plate | 10 days | 35 days | Paresthesia |
| Patient 3 | Inj cephaperazone + metronidazole | Debridement, curettage, extraction, saucerisation, sequestrectomy | 7 days | 8 days | None |
| Patient 4 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, decortication | 10 days | 10 days | Wound break down, Oro antral fistula |
| Patient 5 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage | 5 days | 6 days | None |
| Patient 6 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction | 10 days | 22 days | None |
| Patient 7 | Inj amoxicillin + clavulanic acid and metronidazole | Sinus tract excision, decortication | 5 days | 7 days | None |
| Patient 8 | Inj amoxicillin + clavulanic acid + metronidazole and gentamycin | Debridement, curettage | 8 days | 7 days | None |
| Patient 9 | Inj ampicillin + cloxacillin + metronidazole | Debridement, curettage, extraction | 8 days | 18 days | Paresthesia |
| Patient 10 | Inj amoxicillin + clavulanic acid + metronidazole and gentamycin | Debridement, curettage, extraction, sequestrectomy | 8 days | 10 days | None |
| Patient 11 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage | 7 days | 9 days | None |
| Patient 12 | Inj Taximax, metro, genta | Debridement, curettage, extraction, sequestrectomy | 10 days | 12 days | Paresthesia |
| Patient 13 | Inj cefuroxime + metronidazole | Debridement, curettage, extraction | 10 days | 45 days | None |
| Patient 14 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, sec closure of fistula | 7 days | 10 days | None |
| Patient 15 | Inj amoxicillin + clavulanic acid and metronidazole | Curettage & sinus tract excision | 8 days | 14 days | None |
| Patient 16 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction, sequestrectomy | 9 days | 22 days | Wound break down, Oro antral fistula |
| Patient 17 | Inj amoxicillin + clavulanic acid + metronidazole and gentamycin | Debridement, curettage, extraction, sequestrectomy, buccal pad fat used for closure of fistula | 9 days | 9 days | None |
| Patient 18 | Inj amoxicillin + clavulanic acid + metronidazole and gentamycin | Debridement, curettage, extraction, sequestrectomy | 7 days | 23 days | None |
| Patient 19 | Inj amoxicillin + clavulanic acid +metronidazole and gentamycin | Debridement, curettage, extraction, sequestrectomy | 8 days | 11 days | None |
| Patient 20 | Inj ceftazidine 1 g-TID, Tab Ketoconazole | Debridement, curettage, extraction, sequestrectomy | 10 days | 30 days | Persistent pus discharge |
| Patient 21 | Inj ciprobid + metronidazole | Debridement, curettage, extraction, sequestrectomy, decortication | 7 days | 8 days | None |
| Patient 22 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction, sequestrectomy, ORIF with SS recon plate | 9 days | 15 days | None |
| Patient 23 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction, sequestrectomy, decortication | 8 days | 9 days | None |
| Patient 24 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, sequestrectomy | 7 days | 60 days | None |
| Patient 25 | inj clindamycin + metronidazole + gentamycin | Debridement, curettage, extraction, sequestrectomy, decortication | 7 days | 11 days | None |
| Patient 26 | Inj amoxicillin + clavulanic acid + metronidazole and gentamycin | Debridement, curettage, extraction, sequestrectomy | 8 days | 15 days | None |
| Patient 27 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage, extraction, sequestrectomy | 9 days | 24 days | None |
| Patient 28 | Inj amoxicillin + clavulanic acid + metronidazole and gentamycin | Debridement, curettage, extraction, sequestrectomy | 7 days | 23 days | Wound break down, Oro antral fistula |
| Patient 29 | Inj amoxicillin + clavulanic acid and metronidazole | curettage & sinus tract excision | 8 days | 14 days | None |
| Patient 30 | Inj amoxicillin + clavulanic acid and metronidazole | Debridement, curettage | 7 days | 9 days | None |
| Patient 31 | inj clindamycin + metronidazole + gentamycin | Debridement, curettage, extraction, sequestrectomy | 5 days | 6 days | None |
In 5 patients who were diagnosed with fungal osteomyelitis of maxilla an additional separate antifungal protocol was used wherein Amphotericin B 50 mg dissolved in 500 ml Normal saline @ 0.5 mg/kg intra venous (after test dose) was given. Regular monitoring of the blood urea, creatinine levels for these patients was done while giving this particular drug as it is known to cause renal toxicity. Pre-operatively 3 doses of this drug was given with a gap of 2 days in between the doses as recommended by the physician. Post operatively these patients were followed up with fluconazole 400 mg/day orally for 6 weeks.
The definitive treatment was commenced once the underlying systemic condition had been stabilized. A conservative surgical approach was used in all the cases which mainly included debridement, curettage and extraction of the involved teeth as shown in Table 3. In all the patients with maxillary osteomyelitis, alginate impressions were made prior to the surgery in order to facilitate in making a feeding plate.
In 16 patients sequestrectomy was done, decortication in 7 patients and saucerization in 2 patients. Out of 4 patients with pathological fracture of the mandible, reconstruction plate was used in 2 patients. The reconstruction plate was mainly used for additional stability of the fractured mandible and proper care was taken so that the screws were directed away from the infection site (Fig. 1). In the other two patients, Intermaxillary fixation was done as the patients were not willing for open reduction &internal fixation (ORIF).
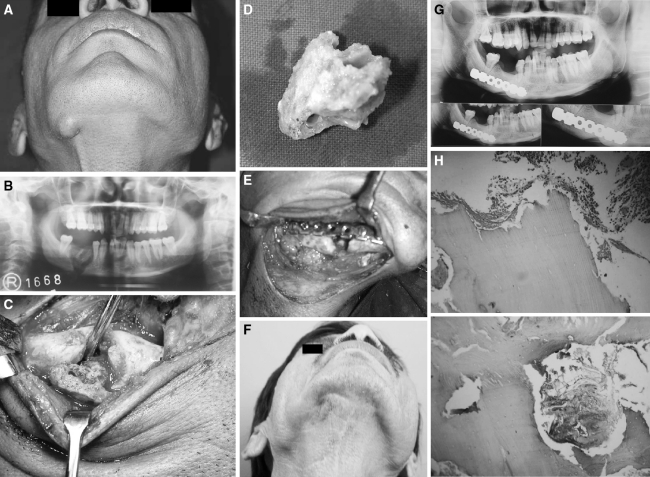
Fig. 1.
a Patient 1 Extra oral swelling in relation to osteomyelitic right mandible. b Patient 1 OPG showing Sequestrum in the right mandible. c Patient 1 Intra-operative view of the involved right mandible. d Patient 1 Sequestrum. e Patient 1 Fixation of the pathological fracture with stainless steel reconstruction plate: H & E ×100 showing dead bone and chronic inflammatory cells H & E ×100 showing dead bone & chronic inflammatory cells. f 8 years follow up showing good extra oral healing. g Patient 1 OPG 8 years post operative showing excellent bone healing at the pathological fracture site
We tried to achieve primary closure in most of our patients. In 1 patient with osteomyelitis of maxilla, buccal pad of fat was used to close the maxillary defect. In most of our patients with maxillary osteomyelitis, the defect that was formed after debridement, curettage& sequestrectomy, was covered with a maxillary feeding plate (Fig. 2).
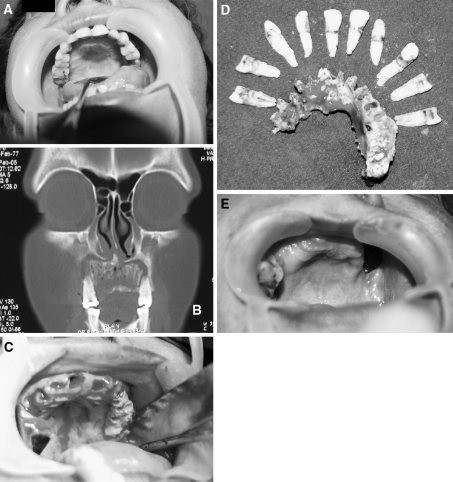
Fig. 2.
a Patient 2 Intra Oral picture of a patient with Maxillary Osteomyelitis. b Coronal section of the CT scan showing Osteomyelitic Maxilla. c Intra operative view after extraction of teeth showing the necrosed bone. d Sequestrum with the extracted teeth. e Intra Oral view showing well healed Maxillary Osteomyelitis with Oro Antral Fistula 1.5 year post operative
Hospital Stay
The post-operative care for these patients mainly consisted of continuing with the antibiotic therapy, intra oral irrigations & dressings. The duration of the antibiotic course mainly depended on the clinical signs & symptoms. The mean duration of intra-venous post operative antibiotics was 7 days following which the patients were on oral antibiotics for 5 days.
The surgical site was irrigated with povidine-iodine & chlorhexidine mouth washes diluted with normal saline.
Number of days the patients admitted in the hospital was also calculated and shown in the Table 3. Depending on the recovery, patients were discharged and their hospital stay ranged from 5 days to 55 days. The mean hospital stay was 13 days.
Follow Up
As a protocol all patients were recalled for follow up 1 week after their discharge from the hospital. They were further recalled after every 1 month for the first 6 months followed by once in 3 months for 1 year and later once in 6 months for the next 3 years. At the end of 12 months we had 26 out of 31 patients and at the end of 3 years 15 patients were available for follow up. We had 5 patients for follow up for more than 5 years. Our mean follow up period was 1 year 11 months.
During the follow up, patients were screened for their general medical status, wound healing and any other persisting symptoms like pus discharge, fistulas, parasthesia etc. Appropriate conventional radiographs (OPG, PNS view) were performed & they were compared with the pre-op radiographs to assess the resolution of condition.
Of the 31 patients, wound healing was satisfactory with no evidence of complications in 23 patients (74.19%). 8 patients (25.8%) encountered complications following surgery as shown in the Table 3.
Three patients with wound break down in the maxilla were managed conservatively with regular intra oral irrigations and maxillary feeding plates.
One patient with persistent pus discharge was again subjected to thorough debridement. Antibiotics regime were changed and patient had recovered satisfactorily.
Patients with pre and post operative parasthesia were reassured and counseled regarding recovery and kept under observation.
Discussion
Osteomyelitis (OM) of the jaw bones, in the post antibiotic era has become a relatively uncommon pathology [2–5]. The term osteomyelitis although a well established clinical entity represents a wide spectrum of clinical conditions with a common pathology of inflammation and infection of the bone. Although literature is abundant on this condition, most of it is in the form of case series and very few comparative studies are seen. A variety of classifications are present based on different aspects of the disease, but they lead to a lot of confusion [6].
In our study the diagnosis was based on clinical and radiological criteria. Biopsy was done essentially to rule out any neoplastic process that may mimic Osteomyelitis [1]. We performed Computerized Tomography scan in 6 patients with maxillary osteomyelitis in order to assess the posterior extent of the involvement as it was not evident in the conventional radiographs of these patients.
The other advanced investigative modalities for osteomyelitis includes Radioisotope 99mTc methylene diphosphonate bone scanning which helps in identifying occult areas of involvement. However due to poor resolution this has not proven to be effective as a diagnostic modality. They however have a good role in determining the resolution of the condition [2]. More recently, Positron emission tomography has shown greater promise in mapping out varying margins of metabolic activity to differentiate between normal bone and affected bone [2]. As there were no facilities in and around our unit & also considering the low socio- economic status of our patients, we have not opted for these advanced methods.
In our series the male to female ratio was 2.1:1. These findings are comparable with other studies [7, 8] although the male to female predilection range may vary up to 5.2:1 [3]. The age range of our patients was between 10 and 65 years and the maximum numbers of our patients were in 4th & 5th decades of life. These findings are slightly in contrast with other studies where a more uniform distribution of patients is seen across the various age groups [5–8].
Majority of the patients in our study had a definite odontogenic component (74%) as the source of infection. This was followed by Maxillary sinusitis (16%) & trauma (6.4%) as etiology. One patient had reported with osteomyelitis of mandible following radiation therapy. In the patient who had osteomyelitis of the mandible post radiotherapy, the cause for the occurrence of osteomyelitis was non-healed extracted socket prior to radiotherapy. In this patient, total extraction of all the remaining teeth was done 2 weeks prior to radiotherapy. Non healed socket post radiotherapy had led to chronic suppurative osteomyelitis with pathological fracture of the mandible. Thorough debridement & curettage was done under general anaesthesia. Intermaxillary fixation was done for the pathological fracture.
In 1 patient no definite cause could be identified. Although many bone conditions like Pagets disease, osteopetrosis, pycnodystosis etc. have been implicated as predisposing factors for osteomyelitis [4, 9] but we found no patients having any such predisposing factors.
All of our patients fall in the chronic (>1 month) variety of suppurative osteomyelitis based on the complaints and clinical presentations (Table no 1). It was very difficult to elicit the exact duration of the complaints but features like paresthesia, pus discharge with fistula, bone exposure, pathological fracture etc. suggest a chronic disease process. Patients with no obvious sclerosis of bone even in the absence of pus discharge were categorized into secondary chronic suppurative osteomyelitis [6, 10].
One significant finding in our series was the high incidence of Maxillary Osteomyelitis. Literature suggests that Maxillary osteomyelitis is relatively uncommon in comparison to mandible due to the porous nature of the bone, significant collateral blood flow and thin cortices [2, 4, 5, 10]. In our study the incidence of maxillary: mandibular osteomyelitis is 1.07: 1 as compared to 1:16.5 [5] and 1:6 [3].
Another interesting finding in our series of patients with maxillary osteomyeltis was the involvement of predominantly the palatal bone along with the maxillary alveolar segment .11 out of 16 patients with maxillary osteomyelitis had a definite periodontal pathology & in the remaining 5 patients, there was a history of recurrent maxillary sinusitis. Operative injury or direct infection from the teeth or the antrum is the usual etiological factor for the maxillary osteomyelitis [11]. This was seen in our series of patients. Palatal bone is a thick compact bone with less medullary spaces, getting blood supply predominantly from the palatal mucosa. The possible explanation for a high incidence of maxillary osteomyelitis in our series is the proximity of the palatal bone to the odontogenic source (periodontal) of infection and extension of infection from the maxillary sinus to the palatal bone. This in conjunction with the immuno-compromised status of the patients could probably have led to the increased incidence of Osteomyelitis of the maxillary bone.
22 out of 31 (70.9%) patients had predisposing associated medical problems as shown in Table 1. 14 out of these 22 patients had Diabetes mellitus. The other medical problems which were noted were severe anemia in 5 patients and malnutrition in 1 patient who was also pregnant. In our study there was 45.1% incidence of diabetes among the patients with osteomyelitis of jaws in contrast to 0% incidence in the studies done by Gerard F. Koorbusch, Abbas A.Y. Taher, Michael E. Koury et al. [5, 7, 12]. Role of Diabetes Mellitus as a suppressor of the host immune response is a well established fact. It also may play a significant role in Osteomyelitis of the jaw bones by compromising the vascularity due to arteritis of the smaller vessels. This has to be further verified by doing blood flow studies similar to the ones done by Wannfors and Gazelius [10]. Since the number of patients in our study was small, we were unable to establish a definite correlation between the diabetic status on admission & the course of the disease. However a strong correlation between diabetic status & the incidence of osteomyelitis among our diabetic patients could be estabilished. Only 14% of the patients diagnosed with diabetes were under good control & the rest had moderate to poor control blood sugar levels.
Another significant finding seen in our series was the high degree of association of Diabetes mellitus with Osteomyelitis of the maxilla which accounts to almost 68% of the total maxillary osteomyelitis cases as compared to only 20% of the cases with mandibular osteomyelitis. This finding suggests that apart from reducing the immune defense mechanism Diabetes mellitus may also significantly contribute to Osteomyelitis by altering the vascularity of the region as mentioned earlier. None of the patients in our series were on immunosuppressive therapy or had HIV infection. Significant anemia and malnutrition probably contributed by altering the host immune response of the patients to resist infections.
Culture and sensitivity results (Table 1) failed to demonstrate positive growth in 16 of the 31 patients. This was probably due to the fact that most of the patients who reported to our unit had treatment already started elsewhere and were on long term empirical Antibiotics. 10 of the positive cultures showed infections of a mixed flora (Aerobic & Anaerobic). Although skin pathogens have traditionally been implicated in Osteomyelitis of the jaws most of the studies have shown a mixed floral infection [2, 3, 5, 7, 14–18]. Another interesting finding of our study was the fungal growth that was seen in 5 patients. All these patients had lesions in the maxilla. When a fungal etiology was present the antifungal protocol described above was followed. Fungal culture and isolation of the organisms can take sometimes up to 6 weeks. Irrespective of the fungal isolation the standard protocol was followed with good results.
The management of all our patients started with correction of the underlying systemic predisposing causes as well as trying to motivate the patients to quit the deleterious habits since they also contribute in the rapid progression of this condition [5]. Dehydration was corrected with administration of appropriate fluids. Empirical broad spectrum antibiotic therapy was started for all patients. Various combination of antibiotics used in our patients is listed in Table 3. As seen in literature the main drug of the antibiotic regimen was penicillin derivative [2, 3, 5, 14]. Patients allergic to penicillin were given Clindamycin or third generation Cephalosporin. Metronidazole was administered to all the patients diagnosed with bacterial Osteomyelitis cases to take care of the anaerobic flora. Gentamycin or Amikacin was added when gram negative infection like pseudomonas or klebsiella were seen during Culture sensitivity. The duration of intra-venous post operative antibiotics is listed in Table 3. The duration of post operative antibiotics in our series of patients depended on the response of the patient to the antibiotic therapy. Most of our patients recovered with 8–10 days of antibiotic therapy, in contrast to the long term antibiotic therapy was recommended by other authors ranging from 30 to 195 days [3, 7, 8, 16].
Surgical intervention forms one of the mainstay treatments for the definitive management of osteomyelitis of jaws. It is aimed at providing drainage to the area of infection, removal of sequestrum and other foreign bodies and getting new blood supply to the area. It ranges from simple sequestrectomy, to segmental resection and reconstruction in recalcitrant cases [2]. A conservative surgical treatment approach was used in most of the cases. The procedures performed for the patients in our series are listed in Table 3. For the patients with pathological fractures of the mandible, reconstruction plates were used in 2 patients because Rigid internal fixation along with debridement gives satisfactory results in Osteomyelitis [12]. Care was taken to keep minimal metal work in the infected areas. In the other two cases, intermaxillary fixation was done as the patients were not willing for plate fixation. In cases of maxillary osteomyelitis which resulted in a maxillary defect following surgical treatment leading to Oro antralfistula, we found that the obturators were the favored method of filling the defect [4].
Hyperbaric oxygen therapy, antibiotic containing acrylic beads, micro vascular grafts has given promising results in the management of patients with refractory osteomyelitis [2, 19]. However our patients responded well with signs of resolution to surgical and intravenous antibiotic therapy. Hence we did not find the need for these methods of treatment in our patients.
Duration of the hospital stay ranged from a minimum of 5 to 55 days with a mean hospital stay of 13 days. The prolonged hospital stay was in a patient who had reported to us with a previous history of Radiation therapy to the mandible.
On follow up, the patients were screened both clinically & radiographically to assess the resolution of the osteomyelitis. Clinically we checked for the resolution of all the clinical signs & symptoms the patients had presented. We also advised conventional radiographs (OPG, PNS views) during the follow up period & they were compared with the pre-operative radiographs to assess the resolution of osteomyelitis. All our patients showed satisfactory resolution of the condition by about 6 weeks.
The complication rate in the series of our patients was 25.8%. Majority of the patients had paresthesia. This was unavoidable due to the proximity of the nerve to the infected site. Three patients had wound break down. These patients were managed with local debridement & irrigations with povidone-Iodine, chlorhexidine mouth washes diluted with normal saline.
Conclusion
The results of this retrospective study conclude that in our series of patients there has been definitely a change in the trend with respect to site of occurrence of osteomyelitis, association of diabetes mellitus with osteomyelitis of jaws, antibiotic regime & the surgical management of osteomyelitis. There has been an unusually higher incidence of maxillary osteomyelitis compared to any other previous studies. The cause for high incidence of maxillary osteomyelitis was analyzed in our series of patients.
We have also observed that there is a strong correlation between the diabetic status of the patients with the incidence of osteomyelitis of the jaws. Correction of the underlying systemic contributing medical problems plays a major role in the resolution of osteomyelitis of the jaws. Conservative surgical treatment which includes debridement, curettage, sequestrectomy along with an appropriate antibiotics alone is effective in the treatment of the osteomyelitis of the jaws. But prospective studies with longitudinal follow up of larger number of patients would be desirable to confirm these findings.
References
- 1.Topazian RG, Goldberg MH, Hupp JR. Oral and maxillofacial infections. 4th edn
- 2.Hudson JW. Osteomyelitis of Jaws: a 50-year perspective. J Oral MaxillofacSurg. 1993;51:1294–1301. doi: 10.1016/S0278-2391(10)80131-4. [DOI] [PubMed] [Google Scholar]
- 3.Rangne A, Rudd A. Osteomyelitis of the jaws. Int J Oral Surg. 1978;7:523–527. doi: 10.1016/S0300-9785(78)80068-4. [DOI] [PubMed] [Google Scholar]
- 4.Barry CP, David Ryan C. Osteomyelitis of the maxilla secondary to osteopetrosis: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:12–15. doi: 10.1067/moe.2003.25. [DOI] [PubMed] [Google Scholar]
- 5.Koorbusch GF, Fotos P, Goll KT. Retrospective assessment of osteomyelitis, etiology, demographics, risk factors and management in 35 cases. Oral Surg Oral Med Oral Pathol. 1992;74:149–154. doi: 10.1016/0030-4220(92)90373-X. [DOI] [PubMed] [Google Scholar]
- 6.Baltensperger M, Gratz K, Bruder E, Lebeda R, Makek M, Eyrich G. Is Primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J Cranio-Maxillofac Surg. 2004;32:43–50. doi: 10.1016/j.jcms.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Taher AAY. Osteomyelitis of the mandible in Tehran, Iran analysis of 88 cases. Oral Surg Oral Med Oral Pathol. 1993;76:28–31. doi: 10.1016/0030-4220(93)90288-F. [DOI] [PubMed] [Google Scholar]
- 8.Kim S-G, Jang H-S. Treatment of chronic osteomyelitis in Korea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:394–398. doi: 10.1067/moe.2001.117810. [DOI] [PubMed] [Google Scholar]
- 9.Van Merkesteyn JPhR, Bras J, Vermeeren JIJF, Van Der Sar A, Statius Van Eps LW (1987) Osteomyelitis of the Jaws in pycnodysostosis. Int J Oral Maxillofac Surg 16:615–619 [DOI] [PubMed]
- 10.Wannfors K, Gazelius B. Blood flow in jaw bones affected by chronic osteomyelitis. Br J Oral Maxillofac Surg. 1991;29:147–153. doi: 10.1016/0266-4356(91)90026-2. [DOI] [PubMed] [Google Scholar]
- 11.Macbeth Ronald. Osteomyelitis of the maxilla. J Laryng Otol. 1952;66(18):1369–1370. doi: 10.1017/s0022215100047228. [DOI] [PubMed] [Google Scholar]
- 12.Koury ME, Perrrott DH, Kaban LB. The use of rigid internal fixation in mandibular fractures complicated by osteomyelitis. J Oral Maxillofac Surg. 1994;52:1114–1119. doi: 10.1016/0278-2391(94)90525-8. [DOI] [PubMed] [Google Scholar]
- 13.Springer ING, Wiltfang J, Dunsche A, Lier GC, Bartsch M, Warnke PH, Barth EL, Terheyden H, Russo PAJ, Czech N, Acil Y. A new method of monitoring osteomyelitis. Int J Oral Maxillofac Surg. 2007;36:527–532. doi: 10.1016/j.ijom.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bevin CR, Inwards CY, Keller EE. Surgical management of primary chronic osteomyelitis: a long-term retrospective analysis. J Oral Maxillofac Surg. 2008;66:2073–2085. doi: 10.1016/j.joms.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Donohue WB, Abelardo LM (1970) Osteomyelitis of the jaw: C.M.A journal/october 10,1970/vol 103 [PMC free article] [PubMed]
- 16.Kinnman JEG, Lee HS (1968) Chronic osteomyelitis of the mandible,clinical study of thirteen cases: O.S,O.M & O.P. January, 1968 [DOI] [PubMed]
- 17.Hovi L, Saarinen UM, Donner U, Lindqvist C. Opportunistic osteomyelitis in the jaws of children on immunosuppressive chemotherapy. J Pediatr Hematol Oncol. 1996;18(1):90–94. doi: 10.1097/00043426-199602000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MA, Embil JM, Canosa T. Osteomyelitis of the maxilla caused by methicillin-resistant Staphylococcus aureus. J Oral Maxillofac Surg. 2003;61:387–390. doi: 10.1053/joms.2003.50076. [DOI] [PubMed] [Google Scholar]
- 19.Grime PD, Bowerman JE, Weller PJ. Gentamicin impregnated polymethylmethacrylate (PMMA) beads in the treatment of primary chronic osteomyelitis of the mandible. Br J Oral Maxillofac Surg. 1990;28:367–374. doi: 10.1016/0266-4356(90)90033-H. [DOI] [PubMed] [Google Scholar]