Abstract
Anti-angiogenic therapy leads to devascularization that limits tumor growth. However, the benefits of angiogenesis inhibitors are typically transient and resistance often develops. In this study, we explored the hypothesis that hypoxia caused by anti-angiogenic therapy induces tumor cell autophagy as a cytoprotective adaptive response, thereby promoting treatment resistance. Hypoxia-induced autophagy was dependent on signaling through the HIF-1α/AMPK pathway, and treatment of hypoxic cells with autophagy inhibitors caused a shift from autophagic to apoptotic cell death in vitro. In glioblastomas clinically resistant to the VEGF neutralizing antibody bevacizumab, increased regions of hypoxia and higher levels of autophagy-mediating BNIP3 were found when compared to pre-treatment specimens from the same patients. When treated with bevacizumab alone, human glioblastoma xenografts showed increased BNIP3 expression and hypoxia-associated growth, which could be prevented by addition of the autophagy inhibitor chloroquine. In vivo targeting of the essential autophagy gene ATG7 also disrupted tumor growth when combined with bevacizumab treatment. Together, our findings elucidate a novel mechanism of resistance to anti-angiogenic therapy in which hypoxia-mediated autophagy promotes tumor cell survival. One strong implication of our findings is that autophagy inhibitors may help prevent resistance to anti-angiogenic therapy used in the clinic.
Keywords: autophagy, glioblastoma, angiogenesis, hypoxia
INTRODUCTION
The hypothesis that tumor progression can be curbed by anti-angiogenic agents targeting abnormal tumor vessels has been confirmed by preclinical evidence and clinical trials (1). However, these initial successes were tempered by the failure of angiogenesis inhibitors to produce enduring clinical responses. For example, in clinical trials of vascular endothelial growth factor (VEGF) neutralizing antibody bevacizumab in glioblastoma (GBM), 40–60% of tumors progressed after initially successful treatment (2), consistent with the development of resistance to anti-angiogenic therapy, a state exhibiting a poor prognosis and poor response to available treatments (3). The molecular basis of acquired resistance to anti-angiogenic treatments causing this lack of sustained responses remains undefined. We hypothesized that the devascularization caused by anti-angiogenic therapy increases tumor hypoxia, and that this hypoxia mediates resistance to anti-angiogenic therapy.
Recent reports suggest that hypoxia activates autophagy, a lysosomal degradation pathway which may promote tumor cell survival (4). The mechanisms by which hypoxia induces autophagy need clarification, but the finding that BNIP3, a hypoxia-inducible factor-1α (HIF-1α) downstream target gene, is essential to hypoxia-induced autophagy suggests one possible mechanism (5).
During autophagy, a crescent-shaped structure, the isolation membrane, forms in the cytoplasm and closes around components targeted for destruction, leading to formation of the autophagosome, which fuses with the lysosome to become an autolysosome, leading to enzymatic degradation of autophagosome contents (6). While autophagosomes were initially identified in dying cells, a phenomenon that led to the term “autophagic cell death” to describe a cell death mode distinct from apoptosis, subsequent studies have shown that autophagy can allow cells to cope with stressors by destroying damaged proteins and organelles as a survival-promoting mechanism (7–9). During autophagosome formation, the ATG7 protein is essential for autophagosome membrane expansion (10).
While autophagosomes sequester cytosolic material nonspecifically in a process called nonselective autophagy, additional evidence shows that a process of selective autophagy also occurs in which autophagic degradation of specific protein aggregates or organelles targeted for destruction occurs. Selective autophagy degrading specific proteins is associated with degradation of p62 (11), a protein complex that binds ubiquitinated protein aggregates to target them for degradation, or BNIP3, a marker of autophagic destruction of mitochondria. Nonselective autophagy can involve microtubule-associated protein light chain 3 (LC3), a protein that, after conversion from its cytosolic form LC3-I to its autophagosome membrane-associated form LC3-II, is ultimately degraded by lysosomal enzymes in autolysosomes during nonselective autophagy, causing the total amount of LC3 (LC3-I plus LC3-II) to drop (6).
We report here that increasing tumor hypoxia occurs during anti-angiogenic therapy and increases tumor cell autophagy as a cell survival mechanism, a novel resistance mechanism to anti-angiogenic therapy. We then investigated the role of hypoxia-upregulated pathways in promoting autophagy. We demonstrated the translational impact of this novel mechanism of resistance to anti-angiogenic therapy by showing in animal models that pharmacologic or genetic autophagy disruption prevented hypoxia-associated resistance to anti-angiogenic therapy. Based on the targetable novel mechanism of resistance to anti-angiogenic therapy described here, combining anti-angiogenic therapy with autophagy inhibition is a therapeutic strategy warranting further investigation in malignant cancers like GBMs.
MATERIALS AND METHODS
Cells and reagents
Cell lines and protocols for primary tumor cell isolation are described in Supplementary Methods. 300,000 cells per well were plated in 12-well plates overnight in DMEM with 10% FBS, then cultured under normoxia (5% CO2, 20% O2, 74% N2) or hypoxia (5% CO2, 1% O2, 94% N2) in a humidified O2 Control incubator (Sanyo) never opened during incubation. Cells were incubated for 3, 6, 16 and 24 hours in DMSO (control), bafilomycin A1 (BafA1; 1 nM), 3-methyladenine (3-MA; 1 mM), chloroquine (10 µM), or YC-1 (10 µM) (Sigma). To quantify GFP-LC3 punctae in U373/GFP cells, 5 random 40x fields were photographed and the average percent of cells per field containing over 10 intracellular GFP-LC3 punctate dots was calculated.
Real time RT-PCR
Real time RT-PCR is described in Supplementary Methods.
siRNA transfection
300,000 cells per well in 12-well plates were transfected overnight using Metafectene transfection reagent (Biontex Laboratories) with 20 nM of negative control, HIF-1α, or AMPKα siGENOME SMARTpool siRNAs (Dharmacon, Inc), which combine four siRNAs into a single pool. After culture with fresh medium for 24 hours, knockdown was confirmed by Western blotting.
Short hairpin RNA (shRNA)-mediated ATG7 knockdown
U87MG and SF8557 cells were infected with SMARTvector2.0 shRNA lentiviral particles (Thermo Scientific) expressing non-targeting negative control (S-005000-01) or 3 human ATG7 shRNAs (SH-020112-01, SH020112-02 and SH020112-03) in the presence of 4 µg/ml polybrene. After 48 hours, subcultured cells were selected in 1ug/ml puromycin for one week. Lysates from stably selected cells were assessed for ATG7 expression by Western blotting.
Western blotting
Western blots were performed as described in the Supplementary Methods.
Cell proliferation and apoptosis assays
5,000 cells per well in 96-well plates in triplicate were plated overnight, then treated with DMSO, 3-MA (1 mM) or BafA1 (5 nM) under normoxia or hypoxia for 48 hours. For extended treatment (72 hours), cells were treated with DMSO, BafA1 (1 nM, 2.5 nM), or chloroquine (10 µM or 25 µM). Relative cell numbers were measured using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega). For apoptosis assays, one million cells per well were plated in 6-well plates overnight, then treated with DMSO, 1 mM 3-MA, or 1 nM BafA1 for 24 hours. Apoptotic and necrotic cells were measured using FITC-Annexin V Apoptosis Detection Kits (BD Pharmingen), with FACS performed with a BD FACSCalibur II machine using FlowJo software (Tree Star, Inc.).
Xenografts
Procedures are described in Supplementary Methods. After tumors were established (mean subcutaneous volume=32 mm3 or 7 days post-intracranial implantation), 5 (subcutaneous tumors) or 10 (intracranial tumors) mice per group were randomly allocated to treatment groups (Supplementary Methods). Tumors were measured with calipers twice weekly. Tumor volume was (width)2 X length/2, and fold-growth was relative to treatment day one. Treatment continued until mice reached IACUC euthanasia criteria (2.1 cm maximal dimension or tumor symptoms). To measure tumor oxygenation, some mice received 60 mg/kg pimonidazole (Hypoxyprobe, Inc.) i.p. one hour before euthanasia.
Human tissue and xenograft immunohistochemistry
Paraffin-embedded sections and xenografts were processed as described in Supplementary Methods. Photos were taken with a Zeiss Axioskop 2 and a Zeiss Axiocam color CCD. Staining quantification is described in Supplementary Methods.
Statistics
Two group comparisons of non-normal values were performed using Wilcoxon signed-rank (paired samples, e.g. pre- and post-treatment) or Wilcoxon rank-sum (unpaired) tests. For subcutaneous tumor volumes, initial comparison of 3 or more groups used the Kruskal-Wallis test (non-parametric alternative to ANOVA), followed by subsequent post hoc testing using the Wilcoxon rank-sum test for two group analyses.
Kaplan-Meier analysis was used to compare survival between groups. P< 0.05 was considered significant, except for the post hoc Wilcoxon rank-sum testing, for which a Bonferroni correction for multiple testing required using αk<0.01 (i.e. 0.01=0.05/k where k=5) to define significance. Error bars are standard deviations, except for tumor volumes, whose error bars are standard errors. Experiments were repeated in triplicate with similar results each time, with figures containing representative experiments.
RESULTS
Patient tumors resistant to anti-angiogenic therapy exhibit increased hypoxia compared to before treatment
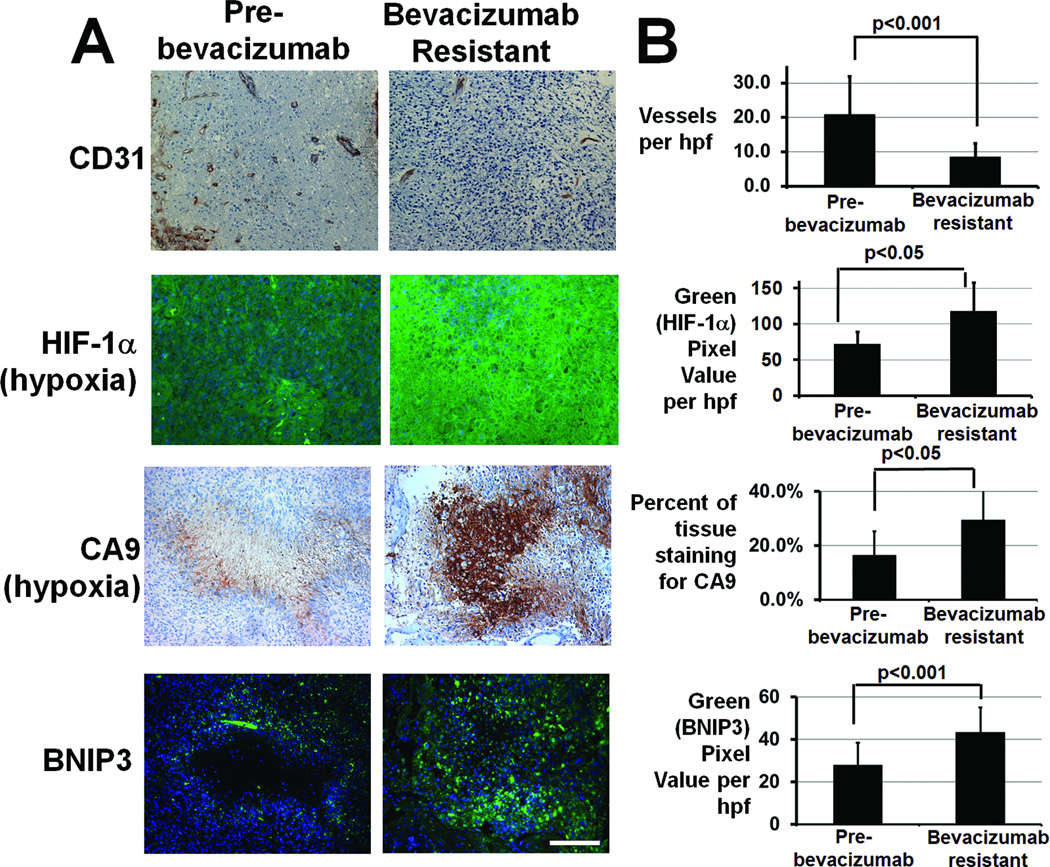
Review of an institutional database of 234 bevacizumab-treated GBMs from 2006–2010 revealed 6 cases meeting the following criteria: (1) after initial radiographic response to bevacizumab as monotherapy (n=2) or combined with topoisomerase inhibitor irinotecan (n=4), these cases exhibited the non-enhancing FLAIR bright growth on MRI seen in bevacizumab-treated GBMs (12, 13); (2) surgical resection of the bevacizumab-resistant tumor occurred within 28 days of last bevacizumab dose; and (3) archived paired pre- and post-treatment tissue was available. Tumor vessel density, assessed by CD31 immunostaining, decreased nearly 60% after bevacizumab resistance compared to pre-treatment specimens from these patients (P<0.001), meanwhile hypoxia-inducible genes HIF-1α and carbonic anhydrase 9 (CA9) (14) immunostaining increased nearly 70% (Fig. 1, P<0.05) and 80% (Fig. 1, P<0.05), respectively. These trends persisted when separating cases into those growing during bevacizumab monotherapy (70% decreased vessel density, 72% increased HIF-1α immunostaining, and 40% increased CA9 immunostaining) versus those growing during bevacizumab plus irinotecan, (50% decreased vessel density, 67% increased HIF-1α immunostaining, and double the CA9 immunostaining), suggesting that, while irinotecan inhibition of HIF-1α expression (15) was more than offset by bevacizumab-induced devascularization and hypoxia-inducible gene expression.
Figure 1. GBMs progressing during anti-angiogenic therapy exhibit decreased vessel density, increased hypoxia, and increased BNIP3 staining compared to pre-treatment GBMs from the same patients.
(A) Representative immunostaining for endothelial marker CD31 (upper row), hypoxia markers HIF-1α (second row; blue=Hoescht nuclear staining; green=HIF-1α), and CA9 (third row), and autophagy-mediating BNIP3 (lower row; blue=Hoescht nuclear staining; green=BNIP3) in this glioblastoma resistant to bevacizumab (right) compared to paired specimens from the same patient before treatment (left). CA9 and BNIP3 stainings are from adjacent sections. (B) Vessel density decreased (P<0.001), hypoxia marker HIF-1α staining increased (P<0.05), hypoxia marker CA9 staining increased (P<0.05), and BNIP3 immunostaining increased (P<0.001) in 6 GBMs after bevacizumab resistance compared to paired pre-treatment specimens. 40x magnification, scale bar=200 µm.
Hypoxia induces autophagy in human GBM cell lines
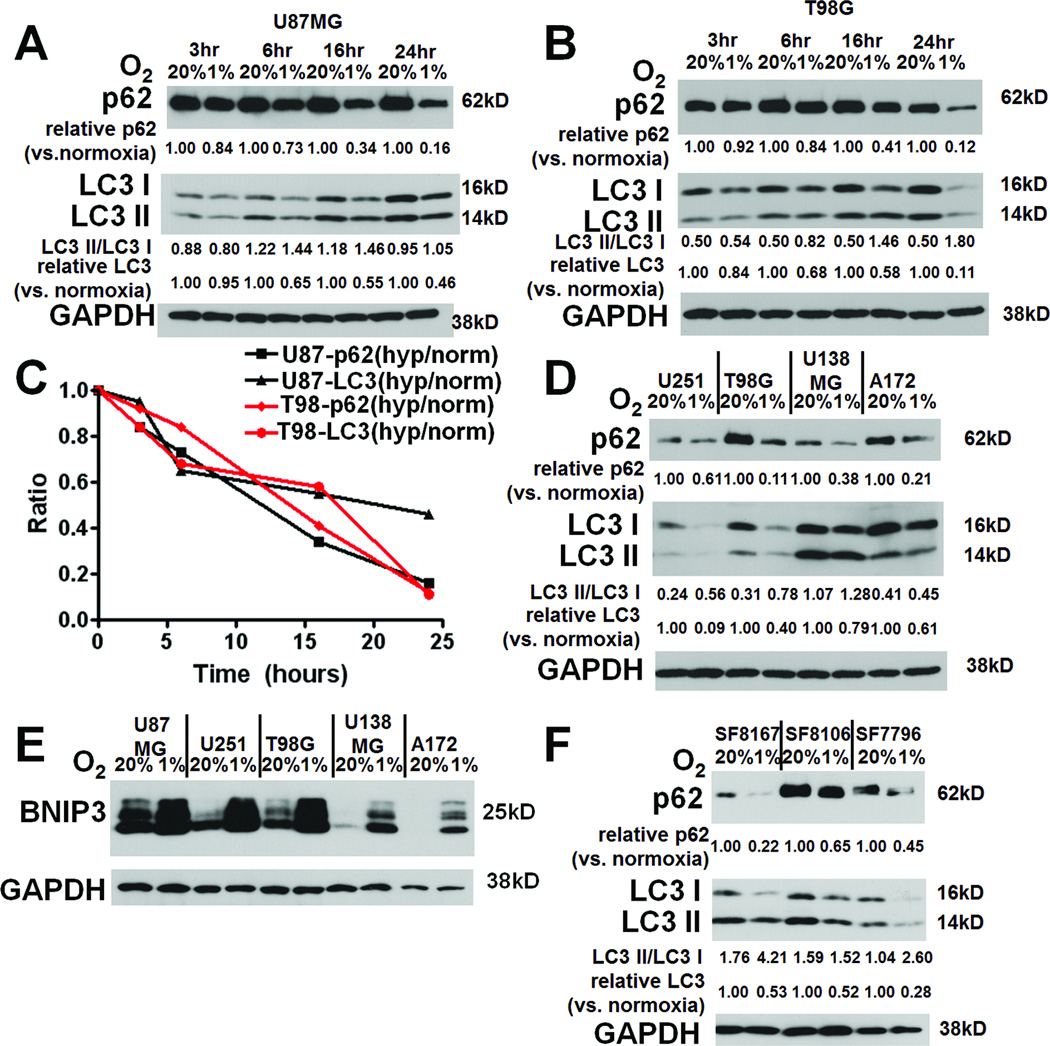
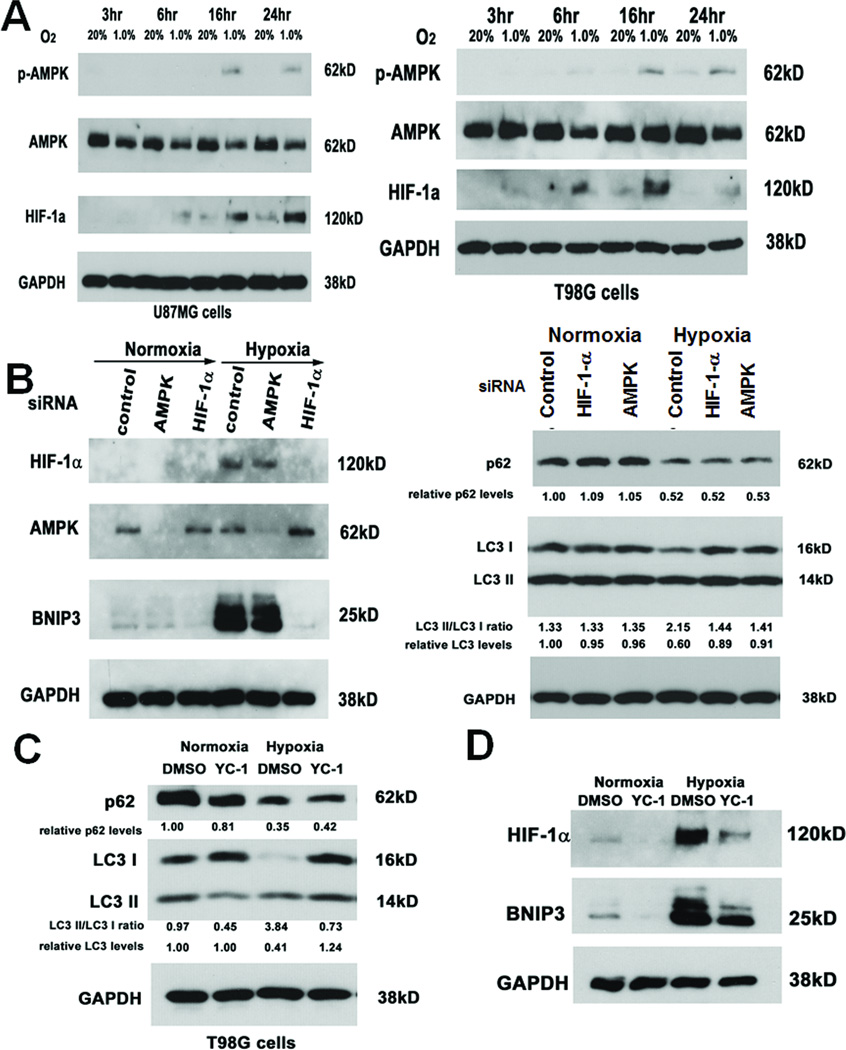
Briefly culturing U87MG and T98G glioma cell lines in hypoxia caused at least two autophagy-associated changes that progressively accumulated at 3, 6, 16, and 24 hours: degradation of total LC3 (LC3-I plus LC3-II) and p62 were seen in U87MG and T98G cells (Figs. 2A-C). Of note, LC3-II expression increased over time in normoxia, consistent with basal autophagy due to metabolite accumulation (16), but this autophagy was clearly increased by hypoxia. Minimal contribution of transcriptional changes to hypoxia-induced autophagy was suggested by the finding that hypoxia-associated alterations in levels of LC3A, LC3B, and p62 transcripts were insignificant (P=0.1–0.9; Fig, S1) and did not correlate with hypoxia duration, consistent with prior reports (17, 18). Similar results were observed in U251, U138MG, A172, and G55 GBM cell lines (Figs. 2D, S2A). LC3-I to LC3-II conversion, a third autophagy-associated change (19), was seen in hypoxic T98G (Fig. 2B), U251 (Fig. 2D), and G55 (Fig. S2A) cells. We further confirmed hypoxia-induced autophagy when we identified that hypoxia upregulated BNIP3 expression in all human GBM cell lines examined (Fig. 2E).
Figure 2. Hypoxia causes autophagy-associated protein changes in GBM cells.
Culturing U87MG (A) and T98G (B) GBM cells in hypoxia for up to 24 hours increased, relative to normoxia degradation of p62 and total LC3 (C), with T98G cells also showing increased conversion of LC3-I to LC3-II in hypoxia (B). Similar findings in 3 other glioma cell lines (U251, U138, A172, and G55) are shown at 24 hours (D). All cell lines also showed hypoxia-induced increased expression of autophagy-mediating BNIP3 (E). (F) Culturing primary glioma cells SF8167, SF8106, SF7796, and SF8244 led to the same hypoxia-induced autophagy-associated changes (p62 degradation, LC3-I to LC3-II conversion, and degradation of total LC3) after 24 hours.
Hypoxia upregulates autophagy in primary GBM cells
Because primary cells might respond differently to hypoxia than cell lines, we studied the ability of hypoxia to induce autophagy-associated protein changes in primary GBM cells from freshly resected human GBMs. Hypoxia induced the same autophagy-associated changes found in cell lines, degradation of p62 and total LC3, in the primary human GBM cells SF8244, SF8167, SF8106, and SF7796, with 2 of these primary human GBM cells also exhibiting hypoxia-induced LC3-I to LC3-II conversion (Figs. 2F and S2A). Because the ATG4 protease modifies LC3 (20), we investigated ATG4 expression in cells exhibiting (T98G, SF8167, and SF7796) or not exhibiting (SF8106) hypoxia-induced LC3-I to LC3-II conversion. ATG4A and ATG4B homologues were not detectable in these cells (data not shown), while ATG4C expression decreased slightly with hypoxia in T98G and the 3 primary glioma cells (Fig. S3), suggesting that ATG4C did not contribute to the hypoxia-induced LC3-I to LC3-II conversion occurring in many glioma cells.
Increased expression of autophagy mediators in human GBMs after bevacizumab resistance
We used immunohistochemistry to identify hypoxic areas and areas that stained positive for autophagy mediator BNIP3 in 6 bevacizumab-resistant GBMs and the paired pre-treatment GBMs from these same patients. The increased hypoxia of these specimens after bevacizumab resistance compared to before was quantified above. While the core of hypoxic areas in these bevacizumab-resistant GBMs was often necrotic, the hypoxic periphery of these necrotic areas stained positive for autophagy-mediating factor BNIP3 (Fig. 1A). Tumors exhibited increased BNIP3 immunoreactivity after bevacizumab failure in a manner reflecting the greater hypoxia seen after resistance to anti-angiogenic therapy (Fig. 1A), with image analysis revealing 55% more BNIP3 immunostaining in the 6 GBMs after bevacizumab resistance compared to tumors from the same patients before bevacizumab treatment (P<0.001; Fig. 1B). These trends persisted when separating cases into those treated with bevacizumab monotherapy (53% increased BNIP3 immunostaining) versus bevacizumab plus irinotecan (57% increased BNIP3 immunostaining).
Autophagy inhibitors disrupt hypoxia-induced GBM autophagy
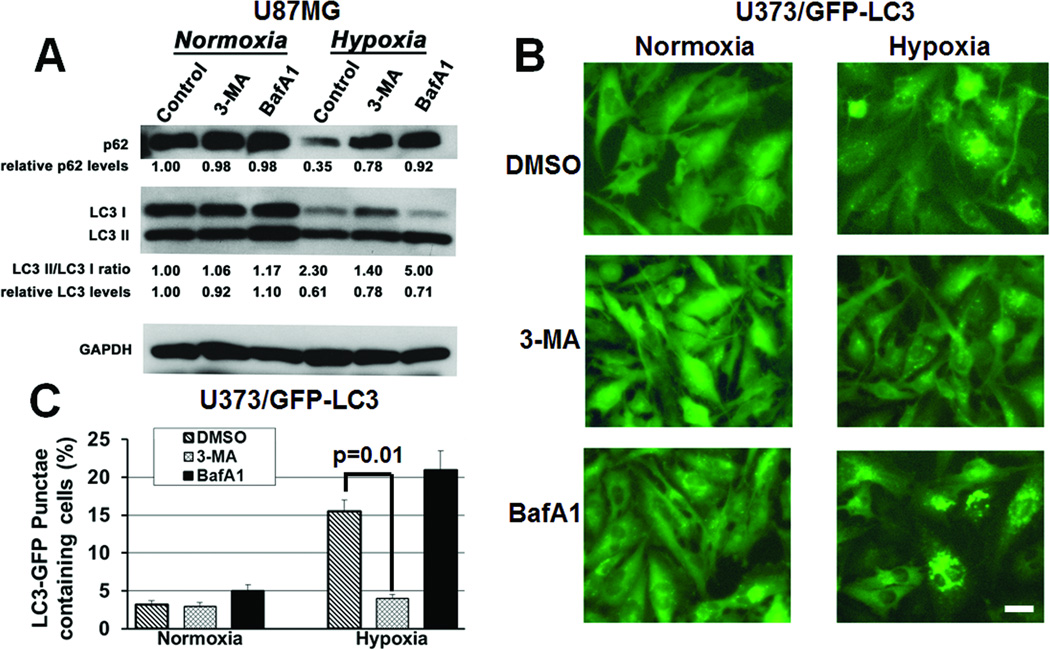
Early autophagy inhibitor 3-methyladenine (3-MA) and late autophagy inhibitor bafilomycin A1 (BafA1) both blocked hypoxia-induced p62 degradation (Fig. 4A). 3-MA inhibited LC3-I to LC3-II conversion, while late autophagy inhibitor BafA1 increased LC3-I to LC3-II conversion (Fig. 3A), reflecting the fact that these inhibitors disrupt autophagy either before (3-MA) or after (BafA1) LC3-I to LC3-II conversion. Similar effects were seen in U373 cells transduced to express a GFP-LC3 fusion protein, with hypoxia increasing autophagy, as assessed by the number of cells with punctate green staining, a marker reduced by early autophagy inhibitors, and Western blotting for free GFP released by autophagic degradation, a marker reduced by early and late autophagy inhibitors (16). Early autophagy inhibitor 3-MA lowered the number of cells with punctate green staining in hypoxia, and late autophagy inhibitor BafA1 maintained the high number of cells with punctate green staining seen in hypoxia (P<0.01; Figs. 3B-C). Similarly, hypoxia increased free GFP identified by Western blotting over 3-fold, with free GFP lowered by early (3-MA) or late (BafA1) autophagy inhibitors, particularly the latter (Fig. S4),
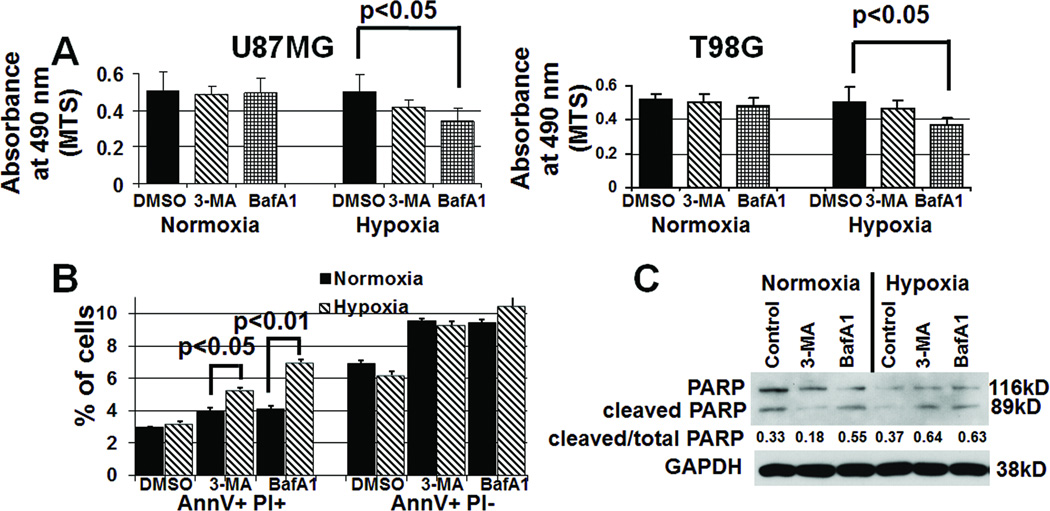
Figure 4. Inhibiting hypoxia-induced autophagy increases cell death.
(A) U87MG and T98G cells in hypoxia for 48 hours exhibited decreased cell numbers, as assessed by absorbance at 490 nm (reflecting number of cells) minus background measured in the MTS assay, when treated with 3-MA (1 mM) or bafilomycin A1 (BafA1; 1 nM) (P<0.05). (B) U87MG cells in hypoxia for 24 hours exhibited an increased percent of AnnexinV+PI+ cells (permeabilized near death cells, leftmost bars) in the presence of 3-MA (P<0.05) or BafA1 (P<0.01) relative to the presence of these inhibitors in normoxia, whilethese inhibitors did not change the percent of AnnexinV+PI− cells (early apoptosis, rightmost bars) in hypoxia (P>0.05). (C) Western blotting revealed increased cleavage of PARP, indicating apoptosis, in hypoxic cells treated with 3-MA or BafA1 for 24 hours, suggesting that autophagy inhibitors were promoting apoptosis.
Figure 3. Autophagy inhibitors block the hypoxia-induced expression of autophagy mediators in glioma cells.
(A) After 24 hours, 3- MA (1 mM) and BafA1 (1 nM) blocked hypoxia-induced p62 degradation in cultured U87MG cells. 3-MA inhibited conversion of LC3-I to LC3-II, while BafA1 increased LC3-I to LC3-II conversion. (B) After 24 hours of hypoxia, U373/GFP-LC3 cells exhibited more punctate green fluorescent staining, consistent with autophagy, which decreased after 3-MA treatment, but remained high after BafA1 treatment. 40x magnification, scale bar=200 µm. (C) 3-MA reduced the percent of cells with over 10 punctate green fluorescent dots (P=0.01).
Inhibiting hypoxia-induced autophagy increases cell death
Next we measured cell survival under hypoxic culturing conditions in the presence of either 3-MA or BafA1. BafA1 significantly decreased the number of viable U87MG and T98G cells in hypoxia (P<0.05; Fig. 4A), with 3-MA having slightly less inhibitory effects on cell viability (P=0.06, Fig. 4A). Having shown a survival-promoting effect of hypoxia-induced autophagy, we characterized the type of cell death occurring in hypoxia when autophagy was inhibited. We performed flow cytometry after using Annexin V (AnnV) and propidium iodine (PI) to label hypoxic U87MG cells that had been treated with and without autophagy inhibitors. In the presence of 3-MA or bafilomycin A, hypoxia significantly increased the number of cells that were AnnV+PI+ (late stage apoptosis) (P<0.01 BafA1, P<0.05 3-MA), while the number of AnnV+PI− cells in early apoptosis did not change (P>0.05) (Fig. 4B). The percentage of total PARP that was active or cleaved, an apoptosis marker, increased in hypoxic cells treated with autophagy inhibitors, with lesser effects seen in normoxia (Fig. 4C), suggesting that the cell death promoted when autophagy was inhibited was apoptotic.
Dependence of various aspects of hypoxia-induced autophagy on HIF-1α or AMPK
Pathways activated by hypoxia in tumor cells that can contribute to autophagy include those mediated by HIF-1α, activated during physiological hypoxia (0.1%–3% O2), or by HIF-1α-independent 5’ adenosine monophosphate (AMP)-activated protein kinase (AMPK), activated during anoxia (≤ 0.01% O2) (4). At 1% oxygen, both HIF-1α and AMPK were activated in U87MG and T98G cells, with HIF-1α activation occurring earlier than AMPK activation (Fig. 5A). Furthermore, siRNA-mediated knockdown of AMPK or HIF-1α blocked hypoxia-mediated LC3-I to LC3-II conversion and total LC3 degradation but neither siRNA affected hypoxia-mediated p62 degradation (Fig. 5B). Similarly, HIF-1α inhibitor YC-1 (21) blocked hypoxia-mediated total LC3 degradation and LC3-I to LC3-II conversion but did not affect hypoxia-mediated p62 degradation (Fig. 5C). We then investigated the role of HIF-1α or AMPK in the hypoxia-mediated BNIP3 expression identified above. Hypoxia-induced BNIP3 expression was reduced with HIF-1α inhibition achieved through HIF-1α siRNA (Fig. 5B) or YC-1 (Fig. 5D). In contrast, AMPK inhibition achieved through AMPK siRNA failed to alter hypoxic induction of BNIP3 (Fig. 5B). Therefore, hypoxia-mediated LC3-I to LC3-II conversion depended on HIF-1α and AMPK, hypoxia-mediate BNIP3 expression depended on HIF-1α not AMPK, and hypoxia-mediated p62 degradation occured independent of these pathways.
Figure 5. Hypoxia upregulates HIF-1α expression and AMPK phosphorylation, which contribute to some aspects of hypoxia-induced autophagy.
(A) U87MG and T98G cells cultured in hypoxia exhibited time-dependent activation of HIF-1α and AMPK (with AMPK activation assessed by detecting phosphorylated AMPK in the first row), with HIF-1α activation occurring before AMPK activation. (B) siRNA-mediated knockdown of AMPK and HIF-1α in hypoxic U87MG cells exhibited reduced LC3-I to LC3-II conversion and reduced total LC3 degradation but neither siRNA affected hypoxia-mediated p62 degradation, while only HIF-1α siRNA reduced hypoxia-induced BNIP3 expression. (C) Similarly, YC-1 (a HIF-1α inhibitor) blocked hypoxia-mediated LC3-I to LC3-II conversion and total LC3 degradation without affecting hypoxia-mediated p62 degradation in T98G cells. (D) In U87MG cells cultured for 24 hours in hypoxia, YC-1 blocked hypoxia-mediated BNIP3 upregulation.
Combined inhibition of autophagy and angiogenesis exerts a potent antitumor effect in vivo
3-MA and BafA1 are typically used as autophagy inhibitors in vitro (22), but chloroquine or hydroxyl-chloroquine, late autophagy inhibitors like BafA1, are used to inhibit autophagy in vivo (23), partly because they are the only FDA-approved autophagy inhibitors. Like BafA1, chloroquine blocked hypoxia-induced p62 degradation, but by blocking autophagy after LC3-I to LC3-II conversion, caused more LC3-I to LC3-II conversion to occur in cultured U87MG, GBM39, and G55 glioma cells (Figs. S5A and S2B), and decreased the viability of U87MG (P<0.05, Fig. S5B) and G55 (P<0.05, Fig. S2C) in hypoxia compared to normoxia. We also examined chloroquine’s effect on BNIP3 expression in 5 cell lines and xenograft-derived cells and found that, while hypoxia increased BNIP3 expression in all cells, chloroquine minimally affected BNIP3 expression under normoxia or hypoxia (Fig. S5C), consistent with prior in vitro reports (45), and suggesting that late autophagy inhibitor chloroquine exerted its effects downstream of BNIP3 upregulation.
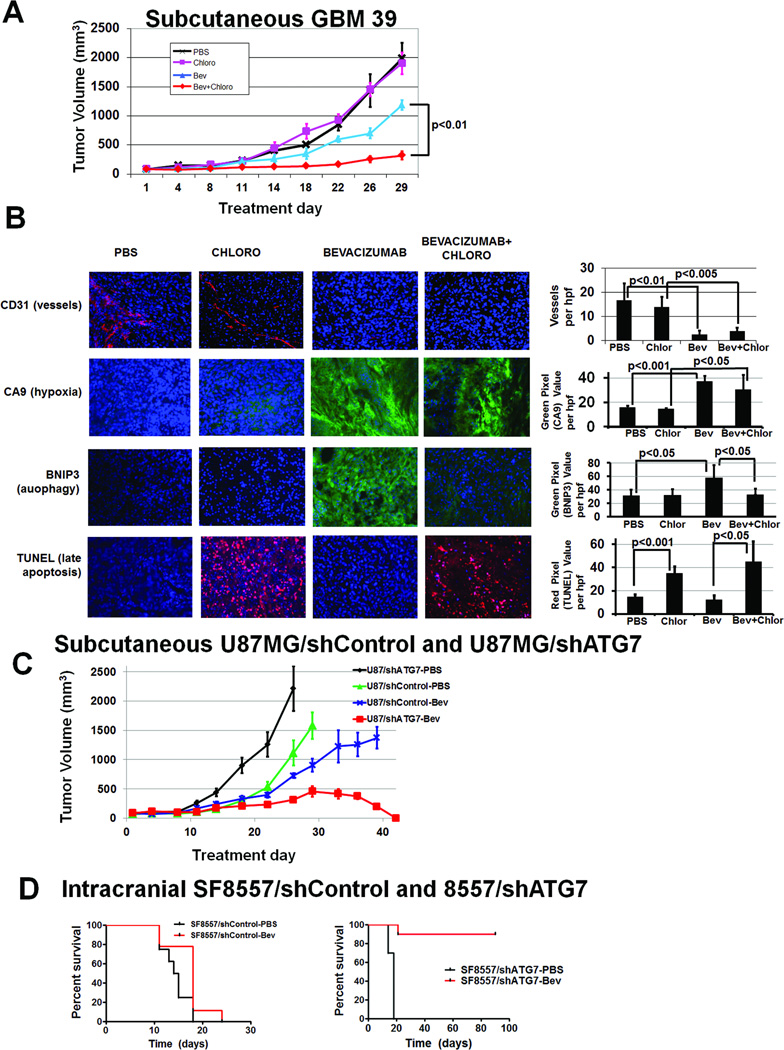
We then investigated whether chloroquine counteracted the survival-promoting effects of hypoxia-induced autophagy caused by anti-angiogenic treatment by treating subcutaneous tumors derived from GBM39 primary glioma cells with autophagy inhibitor chloroquine and/or anti-angiogenic agent bevacizumab. After 4 weeks, tumor volumes differed between the 4 treatment groups (P<0.05) and, compared to PBS, neither chloroquine nor bevacizumab inhibited tumor growth (P=0.3–0.8). Combined therapy (bevacizumab+chloroquine) inhibited tumor growth in a prolonged and significant manner versus either agent alone (P<0.01 bevacizumab vs. bevacizumab+chloroquine; P<0.005 chloroquine vs. bevacizumab+chloroquine) (Fig. 6A). Bevacizumab-treated tumors, with or without combined chloroquine, exhibited 4- to 6-fold reduced vessel density (P<0.01) and over double increased hypoxic area (P<0.05), compared to PBS-treated tumors or tumors treated with chloroquine monotherapy (Fig. 6B), confirming that anti-angiogenic therapy induced devascularization and hypoxia. While bevacizumab monotherapy increased BNIP3 expression nearly 2-fold over than PBS- or chloroquine-treatment (P<0.05), adding chloroquine to bevacizumab reduced BNIP3 expression to levels comparable to PBS or chloroquine-treated tumors (P<0.05; Fig. 6B). Cell death in these xenografts was characterized using TUNEL staining to detect cells in late apoptosis, and staining increased over 2-fold in chloroquine-treated xenografts compared to PBS-treated xenografts (P<0.01) and nearly 4-fold in bevacizumab plus chloroquine-treated xenografts compared to bevacizumab-treated xenografts (P<0.05; Fig. 6B).
Figure 6. Autophagy inhibitor chloroquine combined with bevacizumab inhibits GBM39 tumor growth in vivo.
(A) Subcutaneous tumors in athymic mice were treated with PBS, chloroquine, bevacizumab, and chloroquine plus bevacizumab. After 4 weeks, there was significantly different tumor volumes amongst groups (P<0.05). Compared to PBS (black), neither chloroquine (pink) nor bevacizumab (blue) inhibited tumor growth (P=0.3–0.8). Combined therapy with bevacizumab and chloroquine (red) inhibited tumor growth in a prolonged and statistically significant manner relative to either agent alone (P<0.01 bevacizumab vs. bevacizumab+chloroquine; P<0.005 chloroquine vs. bevacizumab+chloroquine). . (B) Vessel density (CD31 staining, red) decreased in bevacizumab-treated tumors (P<0.01). Hypoxia (CA9 staining, green) increased in bevacizumab-treated tumors (P<0.05). BNIP3 expression (green) increased with bevacizumab treatment (P<0.05), an increase eliminated by adding chloroquine to bevacizumab (P<0.05). TUNEL staining (red) increased in chloroquine-treated tumors (P<0.05). DAPI nuclear counterstaining is blue. Bevacizumab plus chloroquine-treated tumors were small enough that the entire tumor fit one field of view. 20x magnification,. scale bar=200 µm. (C) When subcutaneous U87MG/shControl and U87MG/shATG7 xenografts were treated with PBS or bevacizumab, U87MG/shATG7 tumors completely regressed with bevacizumab treatment (P<0.001), while U87MG/shControl xenografts were minimally responsive (P=0.8). (D) Intracranial SF8557/shATG7 xenografts exhibited 90% long-term survival with bevacizumab treatment, while PBS treatment of intracranial SF8557/shATG7 xenografts led to 18 day median survival (P=0.003). Intracranial SF8557/shControl xenografts exhibited 15 day median survival with PBS, similar to their 18 day median survival with bevacizumab (P=0.3).
Similar sustained tumor growth inhibition in combined treated tumors versus eventual accelerated growth in bevacizumab-treated tumors was noted in subcutaneous U87MG tumors (P<0.005 for 4 group comparison; P<0.01 bevacizumab vs. bevacizumab+chloroquine; Fig. S6A) and G55 (P<0.001 for 4 group comparison; P<0.01 bevacizumab vs. bevacizumab+chloroquine after 8 and 11 treatment days; Fig. S7A) human glioma cell lines. For U87MG-derived xenografts, prolonged treatment of the bevacizumab monotherapy and bevacizumab plus chloroquine groups for 2 additional weeks increased the separation between the 2 groups, with bevacizumab-treated tumors exhibiting an increased growth rate, and the combined treatment tumors exhibiting sustained growth suppression (P<0.01; Fig. S6B). Immunohistochemistry of treated U87MG xenografts revealed similar findings as seen with GBM39 – decreased vessel density and increased hypoxia in bevacizumab-treated xenografts, increased BNIP3 expression in bevacizumab-treated xenografts, and increased TUNEL staining in chloroquine-treated xenografts (Fig. S6C). Western blot of protein from subcutaneous U87MG tumors revealed increased LC3-I to LC3-II conversion after bevacizumab treatment, consistent with autophagy, and after chloroquine treatment, consistent with our in vitro data reflecting the fact that chloroquine is a late autophagy inhibitor (Fig. S6D).
Another patient specimen-derived subcutaneous xenograft, SF8244, exhibited similar sustained lack of growth in combined treated tumors versus eventual accelerated growth in bevacizumab-treated tumors (P<0.01 for 4 group comparison; Fig. S7B). Delayed chloroquine addition to bevacizumab-treated SF8244 tumors that had reached volumes averaging 400 mm3 reduced tumor volume while bevacizumab-treated tumors continued exponential growth (P<0.001; Fig. S7B), suggesting that inhibiting autophagy upon initiation of resistant growth could still suppress anti-angiogenic therapy resistance. Chloroquine alone did not affect tumor growth compared to PBS in any xenografts (P=0.4–0.7).
Knockdown of essential autophagy gene ATG7 promotes bevacizumab responsiveness in vivo
Because chloroquine could exert non-specific effects, to more precisely define the contribution of autophagy to anti-angiogenic therapy resistance, we engineered U87MG and SF8557 glioma cells to stably express 3 different shRNAs targeting autophagy-mediating gene ATG7 (Fig. S8A). Cells expressing the shRNA causing greatest ATG7 knockdown exhibited inhibition of two hypoxia-mediated autophagy-associated protein changes, p62 degradation and LC3-I to LC3-II conversion (Fig. S8B). We treated subcutaneous tumors derived from U87MG/shControl and U87MG/shATG7 cells, and intracranial tumors derived from SF8557/shControl and SF8557/shATG7 cells with PBS or bevacizumab. While subcutaneous U87MG/shControl (Fig. 6C) and intracranial SF8557/shControl (Fig. 6D) tumors exhibited no response to bevacizumab (P=0.3–0.8), all subcutaneous U87MG/shATG7 tumors regressed to cure (P<0.001; Fig. 6C) and intracranial SF8557/shATG7 tumors exhibited 90% long-term survival (Fig. 6D) with bevacizumab treatment (P=0.003). Immunostaining subcutaneous and intracranial shRNA-transduced tumors except for bevacizumab-treated subcutaneous U87MG/shATG7 tumors, which were cured, revealed that bevacizumab decreased vascularity and increased hypoxia in shControl- and shATG7-transduced ectopic and orthotopic tumors (P<0.05; Fig. S9), consistent with our in vivo results with other bevacizumab-treated tumors. BNIP3 expression increased with bevacizumab treatment of shControl- and shATG7-transduced tumors (P<0.05, Fig. S9), with the former consistent with our other in vivo results and the latter consistent with a prior report (24).
DISCUSSION
Cells exposed to various stressors undergo a process of self-digestion known as autophagy, during which cytoplasmic cargo sequestered inside double-membrane vesicles are delivered to the lysosome for degradation. Several in vitro studies suggest that, while autophagy initially prevents cancer cell survival, once a tumor develops, autophagic self catabolization of damaged organelles promotes cell survival by allowing tumor cells to survive the hypoxia and the nutrient and growth factor deprivation (7–9) found in the tumor microenvironment. Suggestion that autophagy promotes tumor cell survival in vivo comes from the correlation of immunostaining for autophagy-promoting BNIP3 with poor cancer survival (25, 26). Several cancer therapies induce autophagy (27–29), and the autophagic response to some treatments is cytoprotective (30).
Because of the failures of conventional DNA damaging chemotherapy, anti-angiogenic therapy has been investigated, with efficacy demonstrated in several cancer clinical trials. However, this efficacy is often transient with acquired resistance to anti-angiogenic therapy common (31). While anti-angiogenic therapy can transiently normalize structural and functional abnormalities in tumor vessels (32), the long-term effect of anti-angiogenic therapy is tumor devascularization, which ultimately worsens tumor hypoxia.
We hypothesized that hypoxia, as occurs after anti-angiogenic therapy (33), would promote autophagy as a cytoprotective adaptive mechanism. Others have shown that hypoxia upregulates autophagy-associated factors, like BNIP3 (34), a finding supported by the identification of BNIP3 expression in perinecrotic regions of patient tumor specimens (35), but whether the response is cytoprotective and which pathways are involved remain undetermined.
The cytoprotective nature of autophagy during hypoxia induced by anti-angiogenic therapy was verified by our in vitro data showing decreased survival of cells treated with autophagy inhibitors in hypoxic conditions, particularly with late autophagy inhibitors, and more so at 72 hours (Fig. S5B) than 48 hours (Fig. 4A) and our in vivo data showing increased TUNEL staining in chloroquine plus bevacizumab-treated xenografts compared to bevacizumab-treated xenografts (Figs. 6B and S6C), suggesting an increased number of apoptotic cells during combined treatment. Of note, while chloroquine consistently exerted anti-tumor effects in hypoxic conditions in vitro and when combined with anti-angiogenic therapy in vivo, it promoted tumor growth, albeit in a manner not quite statistically significant, in normoxic U87MG cells (Fig. S5B) and as monotherapy compared to PBS in G55 xenografts (Fig. S7A). Similarly, in addition to potentiating the response to anti-angiogenic therapy, ATG7 knockdown caused faster in vivo growth of PBS-treated tumors compared to wild-type tumors. These findings illustrate the dual functions of autophagy – a tumoricidal effect under normoxic unstressed conditions, such that autophagy inhibition under those conditions can actually promote tumor growth, versus a tumor-protective effect upon exposure to stressors like the hypoxia caused by anti-angiogenic therapy. These dual functions of autophagy suggest that inhibiting autophagy may be of limited clinical value alone but, when utilized with anti-angiogenic therapy, could provide a therapeutic benefit. These findings also suggest that the effect we observed in vivo was not the additive effect of combining 2 antitumor agents but instead reflected the ability of one therapeutic modality, anti-angiogenic treatment, to turn another modality, autophagy inhibition, with mild tumor-promoting effects into a true antitumor strategy.
The tumor response to hypoxia activates several factors, including HIF-1α-, felt to activate at moderate hypoxia (0.1%), and HIF-1α-independent AMPK, felt to activate at anoxia (0.01%) (4). We found that at 1% oxygen, a concentration more typical of GBMs than 0.1% or 0.01% (36), both HIF-1α and AMPK were activated, with HIF-1α activated earlier than AMPK, suggesting that, different durations of hypoxia, not just different hypoxia levels, may differentially activate these pathways. Both HIF-1α and AMPK could contribute to autophagy, with mTOR inhibition a possible mechanism (37–40). We found that hypoxia-mediated LC3-I to LC3-II conversion and overall LC3 degradation depended on both HIF-1α and AMPK, hypoxia-mediated BNIP3 expression dependended on HIF-1α not AMPK, and hypoxia-mediated p62 degradation was independent of HIF-1α and AMPK. While LC3 contributes to nonselective autophagy (degradation of bulk cytoplasmic contents including organelles), p62 degradation and BNIP3 expression are more involved in selective autophagy destroying ubiquitinated proteins and mitochondria, respectively. Future studies will need to clarify mediators of hypoxia-inducedp62 degradation.
Interestingly, chloroquine minimally affected BNIP3 expression in our cultured cells, consistent with prior reports using cultured colon carcinoma cells treated with BafA1, another late autophagy inhibitor (41), and suggesting that chloroquine inhibited autophagy downstream of BNIP3 expression. In contrast, chloroquine lowered BNIP3 expression in bevacizumab-treated xenografts. The differences between these in vitro and in vivo results could reflect as yet uncharacterized factors in the microenvironment absent in cultured cells, or could reflect the longer treatment duration tumors were exposed to in vivo compared to in culture, potentially increasing cell death and reducing in vivo BNIP3 expression . Tumors derived from cells transduced to express shRNA targeting essential autophagy gene ATG7 exhibited slightly increased BNIP3 expression, consistent with a prior report in which genetic disruption of ATG7 eliminated autophagy but led to a slight increase in BNIP3 expression that could not trigger autophagy in the setting of ATG7 loss (24).
Our findings are significant because we show that targeting autophagy through pharmacologic or genetic means disrupts anti-angiogenic therapy resistance in vivo. While some of these observations were made in ectopic subcutaneous tumors, because of our findings of the importance of hypoxia in resistance to anti-angiogenic therapy and reports that orthotopic murine intracranial tumors exhibit less hypoxia than ectopic subcutaneous tumors and that the hypoxia of the latter more closely resembles human GBM (42, 43), our findings in subcutaneous tumors should be pertinent to GBM.
Chloroquine, a clinically approved anti-malaria drug, has been studied in a randomized GBM trial combining chloroquine with conventional treatment with a benefit not quite significant (44). Currently over 20 phase I/II cancer clinical trials involving chloroquine or hydroxyl-chloroquine are open nationwide (45). Furthermore, while chloroquine plus anti-angiogenic therapy in our xenografts was not curative, chloroquine exerts numerous non-specific effects, incompletely disrupts autophagy, and achieves maximal plasma concentration (46) 10-fold lower than the concentrations inhibiting hypoxia-induced autophagy in vitro. Thus, our finding that genetic disruption of essential autophagy gene ATG7 dramatically increased response to anti-angiogenic therapy from no response to curative, suggests that long-term evaluation of autophagy inhibitors in treating anti-angiogenic therapy resistance will require more specific and potent autophagy inhibitors currently being developed (45).
Supplementary Material
ACKNOWLEDGEMENTS
Work was supported by funding to MKA from the American Brain Tumor Association, the James S. McDonnell Foundation, the NIH (5K02NS64167-2), and the UCSF Brain Tumor SPORE. AJ is a Howard Hughes Medical Institute Research Fellow.
Footnotes
No conflicts of interest to report.
REFERENCES
- 1.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 2.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 3.Clark AJ, Lamborn KR, Butowski NA, Chang SM, Prados MD, Clarke JL, et al. Neurosurgical management and prognosis of patients with glioblastoma that progress during bevacizumab treatment. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e3182314f9d. [DOI] [PubMed] [Google Scholar]
- 4.Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22:177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 7.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Tsuchihara K, Fujii S, Sugiyama M, Goya T, Atomi Y, et al. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007;67:9677–9684. doi: 10.1158/0008-5472.CAN-07-1462. [DOI] [PubMed] [Google Scholar]
- 10.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. 'Protein modifications: beyond the usual suspects' review series. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 13.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 15.Kamiyama H, Takano S, Tsuboi K, Matsumura A. Anti-angiogenic effects of SN38 (active metabolite of irinotecan): inhibition of hypoxia-inducible factor 1 alpha (HIF-1alpha)/vascular endothelial growth factor (VEGF) expression of glioma and growth of endothelial cells. J Cancer Res Clin Oncol. 2005;131:205–213. doi: 10.1007/s00432-004-0642-z. [DOI] [PubMed] [Google Scholar]
- 16.Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- 17.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, et al. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- 18.Pursiheimo JP, Rantanen K, Heikkinen PT, Johansen T, Jaakkola PM. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene. 2009;28:334–344. doi: 10.1038/onc.2008.392. [DOI] [PubMed] [Google Scholar]
- 19.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 20.Satoo K, Noda NN, Kumeta H, Fujioka Y, Mizushima N, Ohsumi Y, et al. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 2009;28:1341–1350. doi: 10.1038/emboj.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC, et al. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2004;10:5566–5571. doi: 10.1158/1078-0432.CCR-04-0076. [DOI] [PubMed] [Google Scholar]
- 26.Giatromanolaki A, Koukourakis MI, Gatter KC, Harris AL, Sivridis E. BNIP3 expression in endometrial cancer relates to active hypoxia inducible factor 1alpha pathway and prognosis. J Clin Pathol. 2008;61:217–220. doi: 10.1136/jcp.2007.046680. [DOI] [PubMed] [Google Scholar]
- 27.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 28.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–1494. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- 29.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 30.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Keunen O, Johansson M, Oudin A, Sanzey M, Abdul Rahim SA, Fack F, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 35.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–6673. [PubMed] [Google Scholar]
- 36.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, et al. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 38.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, et al. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 39.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 43.Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 44.Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 45.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Augustijns P, Geusens P, Verbeke N. Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur J Clin Pharmacol. 1992;42:429–333. doi: 10.1007/BF00280130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.