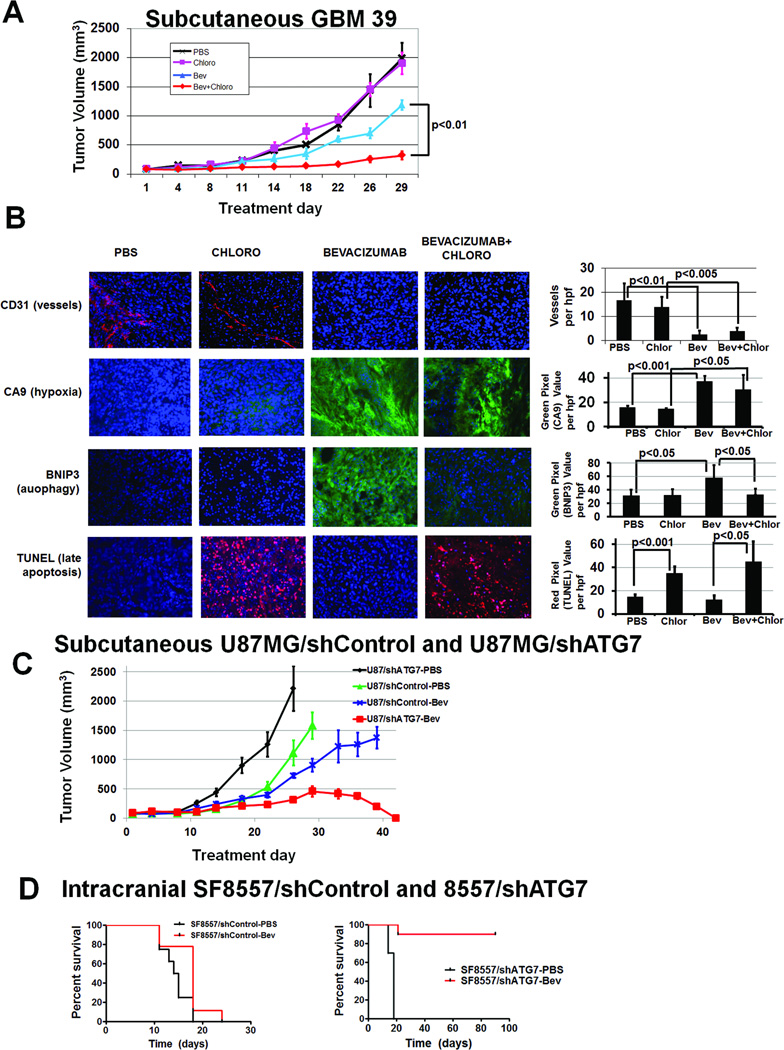
Figure 6. Autophagy inhibitor chloroquine combined with bevacizumab inhibits GBM39 tumor growth in vivo.
(A) Subcutaneous tumors in athymic mice were treated with PBS, chloroquine, bevacizumab, and chloroquine plus bevacizumab. After 4 weeks, there was significantly different tumor volumes amongst groups (P<0.05). Compared to PBS (black), neither chloroquine (pink) nor bevacizumab (blue) inhibited tumor growth (P=0.3–0.8). Combined therapy with bevacizumab and chloroquine (red) inhibited tumor growth in a prolonged and statistically significant manner relative to either agent alone (P<0.01 bevacizumab vs. bevacizumab+chloroquine; P<0.005 chloroquine vs. bevacizumab+chloroquine). . (B) Vessel density (CD31 staining, red) decreased in bevacizumab-treated tumors (P<0.01). Hypoxia (CA9 staining, green) increased in bevacizumab-treated tumors (P<0.05). BNIP3 expression (green) increased with bevacizumab treatment (P<0.05), an increase eliminated by adding chloroquine to bevacizumab (P<0.05). TUNEL staining (red) increased in chloroquine-treated tumors (P<0.05). DAPI nuclear counterstaining is blue. Bevacizumab plus chloroquine-treated tumors were small enough that the entire tumor fit one field of view. 20x magnification,. scale bar=200 µm. (C) When subcutaneous U87MG/shControl and U87MG/shATG7 xenografts were treated with PBS or bevacizumab, U87MG/shATG7 tumors completely regressed with bevacizumab treatment (P<0.001), while U87MG/shControl xenografts were minimally responsive (P=0.8). (D) Intracranial SF8557/shATG7 xenografts exhibited 90% long-term survival with bevacizumab treatment, while PBS treatment of intracranial SF8557/shATG7 xenografts led to 18 day median survival (P=0.003). Intracranial SF8557/shControl xenografts exhibited 15 day median survival with PBS, similar to their 18 day median survival with bevacizumab (P=0.3).