Figure 1.
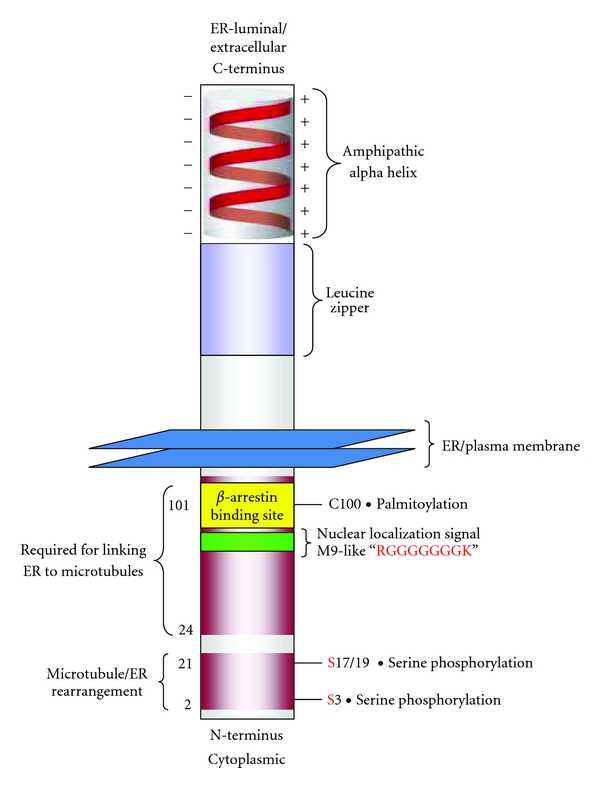
CKAP4 domains. CKAP4 is a 63 kDa, oligomeric, type II, single-pass TM domain protein that is palmitoylated and phosphorylated. The luminal/extracellular domain contains an amphipathic alpha helical region (506-KVQEQVHTLLSQDQA QAARLPPQDFLDRLSSLDNLKASVSQVEADLKMLRTAVDSLVAYSV KIETNENNLESAKGLLDDLRNDLDRLFVKVEKIHEKV-602) that is important for oligomerization and a leucine zipper (468-LASTVRSLGETQLVLYGDVEELKRSVGELPSTVESL-504). Together, these regions are homologous to the DNA-binding domain of bZIP transcription factors. The cytoplasmic, N-terminus contains a cysteine residue adjacent to the TM domain at position 100 that is palmitoylated (a modification that is important for trafficking from the ER to the PM), and three serine residues (S3, S17, and S19) that are required for phosphorylation-dependent binding of CKAP4 to the microtubule cytoskeleton. Two regions in the cytoplasmic N-terminus are required for binding to and bundling microtubules, thereby maintaining the connection between the ER and the cytoskeleton [3]. There is also a PQ protein-protein interaction domain (49-PHPQQHPQQHPQNQ-63) and a putative glycine-rich nuclear localization signal sequence (65-GKGGHRGGGGGGGK-79).
