Figure 5.
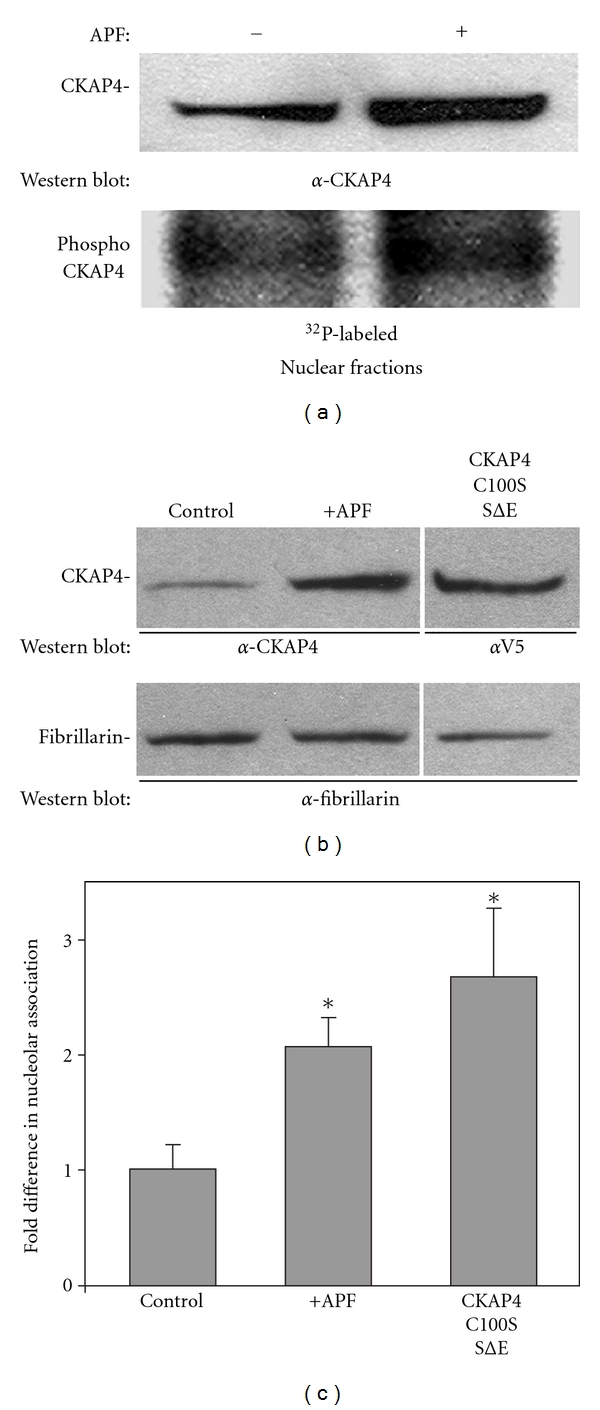
Nuclear CKAP4 is phosphorylated following APF-induced translocation. (a) CKAP4 in the nuclear fraction was phosphorylated to the same degree in APF-treated and control cells as demonstrated by metabolic labeling with γ 32P-ATP. The ratio of the CKAP4 phosphorylation signal over the corresponding CKAP4 Western Blot signal was approximately the same for both (1.00 versus 0.975; APF treated versus control). HeLa cells at ~80% confluence in 10 cm dishes were serum starved for 3 hours then 150 μCi γ 32P-ATP was added to each dish for 1 additional hour. Then the cells were either exposed to APF (20 nM; 24 hours) or left in serum-free medium (control) for 24 hours. At the end of 24 hours, the cells were harvested, washed three times in ice-cold PBS, and the nuclear and cytoplasmic fractions were isolated using the NE-PER (Pierce). Equal quantities of each fraction were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was incubated overnight at 4°C in α-CKAP4 antibody (1 : 500) in TBST and 1% milk, washed and incubated for six hours at 4°C in a-mouse, HRP-conjugated secondary antibody (Pierce; 1 : 20,000) in TBST and 1% milk. CKAP4 bands were detected by enhanced chemiluminescence (ECL; Pierce; 20 second exposure; left panel). Following the Western Blot, the membrane was rinsed in 1% H2O2 for 1 minute to eliminate the chemiluminescent signal then wrapped in plastic wrap and exposed to film for 12 hours to detect the phosphorylated proteins. Densitometric analysis of the immunoreactive bands was done using ImageJ, and the ratios of phosphorylated to nonphosphorylated CKAP4 were determined. APF increases the association of CKAP4 with nucleoli. (b) and (c) Western Blot analysis shows that treatment of HeLa cells with APF (20 nM) increased the association of endogenous CKAP4 with the nucleolar fraction, and that the constitutively depalmitoylated and phosphomimicking CKAP4 mutant, CKAP4C100S/SΔE, associated with the nucleolar fraction to a greater extent than endogenous CKAP4 isolated from APF-treated cells. Nucleoli were isolated using a variation on the method published by Busch and coworkers [20] as described by the Angus Lamond lab (University of Dundee, UK). The nucleolar proteins were separated by SDS-PAGE and Western Blotted for CKAP4 (α-V5 in the case of CKAP4C100S/SΔE) and fibrillarin. The bands were detected on film by ECL and quantified using ImageJ. The CKAP4 signal in each lane of the Western Blot was normalized to the fibrillarin band in the same lane. The normalized value for CKAP4 from control cells was set to 1 and the other values were set relative to control. The values in the graph are means and SD. “*” indicates that the means of the values were significantly different than serum-starved control when evaluated using the students t-test (two-tailed. Serum starved versus APF treated P = 0.018; serum starved versus CKAP4C100S/SΔE P = 0.002; CKAP4C100S/SΔE versus APF treated, P = 0.008; n = 3 for each).
